当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ROS inhibits RORα degradation by decreasing its arginine methylation in liver cancer
Cancer Science ( IF 4.5 ) Pub Date : 2022-09-17 , DOI: 10.1111/cas.15595 Hyuntae Im 1 , Hee-Ji Baek 2, 3 , Eunbi Yang 2, 3 , Kyeongkyu Kim 4 , Se Kyu Oh 5 , Jung-Shin Lee 1 , Hyunkyung Kim 2, 3 , Ji Min Lee 6
Cancer Science ( IF 4.5 ) Pub Date : 2022-09-17 , DOI: 10.1111/cas.15595 Hyuntae Im 1 , Hee-Ji Baek 2, 3 , Eunbi Yang 2, 3 , Kyeongkyu Kim 4 , Se Kyu Oh 5 , Jung-Shin Lee 1 , Hyunkyung Kim 2, 3 , Ji Min Lee 6
Affiliation
|
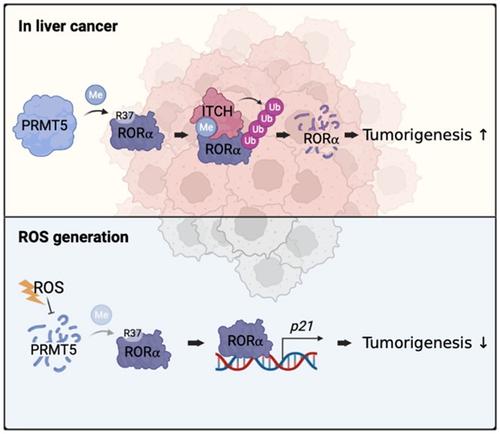
Retinoic acid receptor–related orphan receptor α (RORα) is a transcription factor involved in nuclear gene expression and a known tumor suppressor. RORα was the first identified substrate of lysine methylation–dependent degradation. However, the mechanisms of other post-translational modifications (PTMs) that occur in RORα remain largely unknown, especially in liver cancer. Arginine methylation is a common PTM in arginine residues of nonhistone and histone proteins and affects substrate protein function and fate. We found an analogous amino acid disposition containing R37 at the ROR N-terminus compared to histone H3 residue, which is arginine methylated. Here, we provide evidence that R37 methylation–dependent degradation is carried out by protein arginine methyltransferase 5 (PRMT5). Further, we discovered that PRMT5 regulated the interaction between the E3 ubiquitin ligase ITCH and RORα through RORα arginine methylation. Arginine methylation–dependent ubiquitination-mediated RORα degradation reduced downstream target gene activation. H2O2-induced reactive oxygen species (ROS) decreased PRMT5 protein levels, consequently increasing RORα protein levels in HepG2 liver cancer cells. In addition, ROS inhibited liver cancer progression by inducing apoptosis via PRMT5-mediated RORα methylation and the ITCH axis. Our results potentiate PRMT5 as an elimination target in cancer therapy, and this additional regulatory level within ROS signaling may help identify new targets for therapeutic intervention in liver cancer.
中文翻译:

ROS 通过降低其在肝癌中的精氨酸甲基化来抑制 RORα 降解
视黄酸受体相关孤儿受体 α (RORα) 是一种参与核基因表达的转录因子,也是一种已知的肿瘤抑制因子。RORα 是第一个确定的赖氨酸甲基化依赖性降解底物。然而,发生在 RORα 中的其他翻译后修饰 (PTM) 的机制在很大程度上仍然未知,尤其是在肝癌中。精氨酸甲基化是非组蛋白和组蛋白精氨酸残基中常见的 PTM,它会影响底物蛋白的功能和命运。与精氨酸甲基化的组蛋白 H3 残基相比,我们发现在 ROR N 末端包含 R37 的类似氨基酸配置。在这里,我们提供证据表明 R37 甲基化依赖性降解是由蛋白质精氨酸甲基转移酶 5 (PRMT5) 进行的。进一步,我们发现 PRMT5 通过 RORα 精氨酸甲基化调节 E3 泛素连接酶 ITCH 和 RORα 之间的相互作用。精氨酸甲基化依赖的泛素化介导的 RORα 降解减少了下游靶基因的激活。H2 O 2诱导的活性氧 (ROS) 降低了 PRMT5 蛋白水平,从而增加了 HepG2 肝癌细胞中的 RORα 蛋白水平。此外,ROS 通过 PRMT5 介导的 RORα 甲基化和 ITCH 轴诱导细胞凋亡来抑制肝癌进展。我们的结果加强了 PRMT5 作为癌症治疗中的消除目标,并且 ROS 信号中的这种额外的调节水平可能有助于确定肝癌治疗干预的新目标。
更新日期:2022-09-17
中文翻译:

ROS 通过降低其在肝癌中的精氨酸甲基化来抑制 RORα 降解
视黄酸受体相关孤儿受体 α (RORα) 是一种参与核基因表达的转录因子,也是一种已知的肿瘤抑制因子。RORα 是第一个确定的赖氨酸甲基化依赖性降解底物。然而,发生在 RORα 中的其他翻译后修饰 (PTM) 的机制在很大程度上仍然未知,尤其是在肝癌中。精氨酸甲基化是非组蛋白和组蛋白精氨酸残基中常见的 PTM,它会影响底物蛋白的功能和命运。与精氨酸甲基化的组蛋白 H3 残基相比,我们发现在 ROR N 末端包含 R37 的类似氨基酸配置。在这里,我们提供证据表明 R37 甲基化依赖性降解是由蛋白质精氨酸甲基转移酶 5 (PRMT5) 进行的。进一步,我们发现 PRMT5 通过 RORα 精氨酸甲基化调节 E3 泛素连接酶 ITCH 和 RORα 之间的相互作用。精氨酸甲基化依赖的泛素化介导的 RORα 降解减少了下游靶基因的激活。H2 O 2诱导的活性氧 (ROS) 降低了 PRMT5 蛋白水平,从而增加了 HepG2 肝癌细胞中的 RORα 蛋白水平。此外,ROS 通过 PRMT5 介导的 RORα 甲基化和 ITCH 轴诱导细胞凋亡来抑制肝癌进展。我们的结果加强了 PRMT5 作为癌症治疗中的消除目标,并且 ROS 信号中的这种额外的调节水平可能有助于确定肝癌治疗干预的新目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号