当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The “burst effect” of hydrogen desorption in MgH2 dehydrogenation
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-09-16 , DOI: 10.1039/d2ta06458h Shuai Dong 1, 2 , Chaoqun Li 1, 2 , Jinhui Wang 1, 2 , Hao Liu 1, 2 , Zhao Ding 3 , Zhengyang Gao 1, 2 , Weijie Yang 1, 2 , Wei Lv 4, 5 , Li Wei 6 , Ying Wu 4, 5 , Hao Li 7
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-09-16 , DOI: 10.1039/d2ta06458h Shuai Dong 1, 2 , Chaoqun Li 1, 2 , Jinhui Wang 1, 2 , Hao Liu 1, 2 , Zhao Ding 3 , Zhengyang Gao 1, 2 , Weijie Yang 1, 2 , Wei Lv 4, 5 , Li Wei 6 , Ying Wu 4, 5 , Hao Li 7
Affiliation
|
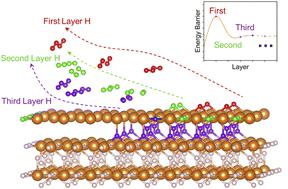
Magnesium hydride (MgH2) is a promising material for solid hydrogen storage due to its superior hydrogen storage capacity. However, its commercial application is inhibited by the sluggish dehydrogenation kinetics resulting from the complex hydrogen migration and desorption processes. Herein, we study the sequential MgH2 dehydrogenation mechanism by analyzing the kinetic and structural changes during the layer-by-layer hydrogen desorption process. Our results obtained by spin-polarized density functional theory calculations with van der Waals corrections (DFT-D3) unveiled an interesting “burst effect” during MgH2 dehydrogenation. We found that the initial dehydrogenation barriers (2.52 and 2.53 eV) are much higher than the subsequent reaction barriers (0.12–1.51 eV). The Mg–H bond analyses by the crystal orbital Hamilton population method indicate that the Mg–H bond strength decreases along the dehydrogenation process. Therefore, the subsequent H migration and hydrogen desorption become significantly easier, showing a “burst effect”. Electronic structure analyses using the electron localization function show that the H vacancy still has a high degree of electronic localization when the first layer of atomic H exists. Furthermore, ab initio molecular dynamics simulations were performed to analyze the kinetic characteristics of MgH2 after surface dehydrogenation to provide more evidence. This identified burst effect provides a theoretical basis for the dehydrogenation kinetics of MgH2 and proposes important guidelines for modifying MgH2-based hydrogen storage materials: promoting the initial dehydrogenation by structural engineering could be the key to facilitating the hydrogen desorption of MgH2.
中文翻译:

MgH2脱氢中氢解吸的“爆发效应”
氢化镁(MgH 2)因其优异的储氢能力而成为一种很有前途的固态储氢材料。然而,由于复杂的氢迁移和解吸过程导致缓慢的脱氢动力学,其商业应用受到抑制。在此,我们通过分析逐层氢解吸过程中的动力学和结构变化来研究顺序MgH 2脱氢机制。我们通过使用范德华校正 (DFT-D3) 的自旋极化密度泛函理论计算获得的结果揭示了 MgH 2期间有趣的“爆发效应”脱氢。我们发现最初的脱氢能垒(2.52 和 2.53 eV)远高于随后的反应能垒(0.12-1.51 eV)。通过晶体轨道汉密尔顿布居法分析 Mg-H 键表明,Mg-H 键强度随着脱氢过程而降低。因此,随后的 H 迁移和氢解吸变得明显容易,呈现出“爆发效应”。使用电子定域函数的电子结构分析表明,当第一层原子H存在时,H空位仍然具有高度的电子定域性。此外,进行从头算分子动力学模拟以分析MgH 2的动力学特性表面脱氢后提供更多证据。这一确定的爆发效应为MgH 2的脱氢动力学提供了理论基础,并为改性MgH 2基储氢材料提出了重要指导方针:通过结构工程促进初始脱氢可能是促进MgH 2氢解吸的关键。
更新日期:2022-09-16
中文翻译:

MgH2脱氢中氢解吸的“爆发效应”
氢化镁(MgH 2)因其优异的储氢能力而成为一种很有前途的固态储氢材料。然而,由于复杂的氢迁移和解吸过程导致缓慢的脱氢动力学,其商业应用受到抑制。在此,我们通过分析逐层氢解吸过程中的动力学和结构变化来研究顺序MgH 2脱氢机制。我们通过使用范德华校正 (DFT-D3) 的自旋极化密度泛函理论计算获得的结果揭示了 MgH 2期间有趣的“爆发效应”脱氢。我们发现最初的脱氢能垒(2.52 和 2.53 eV)远高于随后的反应能垒(0.12-1.51 eV)。通过晶体轨道汉密尔顿布居法分析 Mg-H 键表明,Mg-H 键强度随着脱氢过程而降低。因此,随后的 H 迁移和氢解吸变得明显容易,呈现出“爆发效应”。使用电子定域函数的电子结构分析表明,当第一层原子H存在时,H空位仍然具有高度的电子定域性。此外,进行从头算分子动力学模拟以分析MgH 2的动力学特性表面脱氢后提供更多证据。这一确定的爆发效应为MgH 2的脱氢动力学提供了理论基础,并为改性MgH 2基储氢材料提出了重要指导方针:通过结构工程促进初始脱氢可能是促进MgH 2氢解吸的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号