当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid–Gas Phase Equilibria for Small Phenylene-Thiophene Co-Oligomers
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-16 , DOI: 10.1021/acs.jced.2c00459 Carlos F. R. A. C. Lima 1, 2 , José C. S. Costa 1, 3 , Artur M. S. Silva 2 , Adélio Mendes 3 , Luís M. N. B. F. Santos 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-16 , DOI: 10.1021/acs.jced.2c00459 Carlos F. R. A. C. Lima 1, 2 , José C. S. Costa 1, 3 , Artur M. S. Silva 2 , Adélio Mendes 3 , Luís M. N. B. F. Santos 1
Affiliation
|
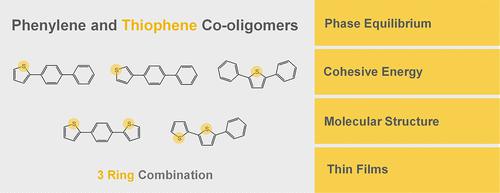
This work reports a comprehensive experimental evaluation of the solid–liquid–gas phase equilibria for five representative phenylene-thiophene co-oligomers (3-ring aromatic compounds having both phenyl and thienyl units). The melting temperatures and corresponding standard molar enthalpies and entropies of fusion were measured by differential scanning calorimetry. The equilibrium vapor pressures of the crystalline solids as a function of temperature were measured by a combined Knudsen/quartz-crystal effusion method, with the consequent derivation of the standard molar enthalpies, entropies, and Gibbs energies of sublimation. The thermodynamic properties of vaporization were estimated from the fusion and sublimation data. The results were analyzed together with the literature data for the corresponding phenylene and thiophene homo-oligomers. The thermodynamic properties of fusion and sublimation exhibited a dependence on ring identity and position that cannot be adequately described by a simple group additivity reasoning. The plot of the Gibbs energy of sublimation as a function of the number of thienyl rings in the co-oligomer showed the existence of two series. Terminal 3-thienyl rings and a linear molecular shape were found to be consistent factors contributing to the stabilization of the crystal phase. The higher melting temperatures and lower volatilities of crystalline 3-thienyl compounds were tentatively explained by the ability of these rings to maximize intermolecular C–H···π interactions independently of the sulfur position. The optical energy gaps, as measured by UV–vis in solution, were found to lie within the values for typical organic semiconductors (<4 eV) and to decrease for co-oligomers containing more 2-thienyl units, following the increased ring–ring planarity of the molecules. The surface morphology of vapor-deposited thin films suggests a stronger tendency of the co-oligomers, if compared to their corresponding homo-oligomers p-terphenyl and terthiophene, to form less amorphous films.
中文翻译:

小亚苯基-噻吩共低聚物的固-液相-气相平衡
这项工作报告了对五种代表性亚苯基-噻吩共低聚物(具有苯基和噻吩基单元的三环芳族化合物)的固-液-气相平衡的综合实验评估。通过差示扫描量热法测量熔化温度和相应的标准摩尔焓和熔化熵。作为温度函数的结晶固体的平衡蒸汽压通过组合的克努森/石英晶体渗出法测量,随后推导标准摩尔焓、熵和升华吉布斯能量。从聚变和升华数据估计汽化的热力学性质。结果与相应的亚苯基和噻吩均聚物的文献数据一起分析。聚变和升华的热力学性质表现出对环身份和位置的依赖性,这不能通过简单的群加性推理来充分描述。作为共低聚物中噻吩环数量的函数的升华吉布斯能量图表明存在两个系列。发现末端 3-噻吩环和线性分子形状是有助于稳定晶相的一致因素。结晶 3-噻吩基化合物较高的熔融温度和较低的挥发性可以通过这些环使分子间 C-H···π 相互作用与硫位置无关的能力得到初步解释。发现溶液中通过紫外-可见光测量的光学能隙位于典型有机半导体的值范围内(< 4 eV)并随着分子的环-环平面度增加而减少含有更多 2-噻吩基单元的共低聚物。气相沉积薄膜的表面形态表明,与相应的均聚物相比,共低聚物的趋势更强对三联苯和三联噻吩,形成较少的无定形薄膜。
更新日期:2022-09-16
中文翻译:

小亚苯基-噻吩共低聚物的固-液相-气相平衡
这项工作报告了对五种代表性亚苯基-噻吩共低聚物(具有苯基和噻吩基单元的三环芳族化合物)的固-液-气相平衡的综合实验评估。通过差示扫描量热法测量熔化温度和相应的标准摩尔焓和熔化熵。作为温度函数的结晶固体的平衡蒸汽压通过组合的克努森/石英晶体渗出法测量,随后推导标准摩尔焓、熵和升华吉布斯能量。从聚变和升华数据估计汽化的热力学性质。结果与相应的亚苯基和噻吩均聚物的文献数据一起分析。聚变和升华的热力学性质表现出对环身份和位置的依赖性,这不能通过简单的群加性推理来充分描述。作为共低聚物中噻吩环数量的函数的升华吉布斯能量图表明存在两个系列。发现末端 3-噻吩环和线性分子形状是有助于稳定晶相的一致因素。结晶 3-噻吩基化合物较高的熔融温度和较低的挥发性可以通过这些环使分子间 C-H···π 相互作用与硫位置无关的能力得到初步解释。发现溶液中通过紫外-可见光测量的光学能隙位于典型有机半导体的值范围内(< 4 eV)并随着分子的环-环平面度增加而减少含有更多 2-噻吩基单元的共低聚物。气相沉积薄膜的表面形态表明,与相应的均聚物相比,共低聚物的趋势更强对三联苯和三联噻吩,形成较少的无定形薄膜。









































 京公网安备 11010802027423号
京公网安备 11010802027423号