Water Research ( IF 11.4 ) Pub Date : 2022-09-14 , DOI: 10.1016/j.watres.2022.119113 Jun Liang 1 , Kexin Chen 1 , Xiaoguang Duan 2 , Ling Zhao 1 , Hao Qiu 1 , Xiaoyun Xu 1 , Xinde Cao 3
|
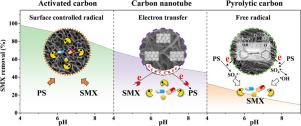
The impacts of pH on purification efficiency can be phenomenal in advanced oxidation processes (AOPs), because solution pH affects persulfate (PS) activation processes. However, consensus has not been reached on the regimes of pH-regulated oxidation in persulfate-based AOPs (PS-AOPs). Particularly, the impacts of pH on carbon-catalyzed generation of radical and nonradical species remain unclear. In this work, we evaluated three typical carbonaceous materials including pyrolytic carbon (PC), activated carbon (AC), and carbon nanotube (CNT) to activate PS for sulfamethoxazole (SMX) degradation within a pH range from 4 to 9. The experiment revealed pH-dependent SMX removal in PC/PS, AC/PS, and CNT/PS, and the kinetics followed an order of pH 4 > pH 7 > pH 9. Solution pH simultaneously affected SMX adsorption and degradation, but the latter was more profound. Chemical quenching experiment, electrochemical measurement, kinetics calculation, and ATR-FTIR tests collectively revealed that high pH was not favorable for both radical and nonradical oxidation. In the PC/PS system, increased pH decreased the amount of phenolic –OH on PC surface, thereby restraining the generation of SO4•− and •OH due to the lack of electron donors. For AC/PS system, elevated pH hindered the interactions between AC and PS, thus suppressing the formation of surface-bound radicals. CNT/PS initiated an electron-transfer pathway, and increased pH reduced the oxidation potential of surface CNT-PS* complex, which was not favorable for nonradical oxidation of adsorbed pollutants. Therefore, outcomes of this work will advance the current knowledge on the intrinsic impacts of pH in PS-AOPs catalyzed by carbonaceous materials for wastewater purification.
中文翻译:

不同碳/过硫酸盐体系中磺胺甲恶唑降解的自由基和非自由基物质的 pH 依赖性生成
在高级氧化工艺 (AOP) 中,pH 值对净化效率的影响可能非常显着,因为溶液 pH 值会影响过硫酸盐 (PS) 活化过程。然而,在基于过硫酸盐的 AOP (PS-AOP) 中 pH 调节的氧化机制尚未达成共识。特别是,pH 对碳催化自由基和非自由基物种生成的影响仍不清楚。在这项工作中,我们评估了三种典型的碳质材料,包括热解碳 (PC)、活性炭 (AC) 和碳纳米管 (CNT),以在 4 到 9 的 pH 范围内激活 PS 降解磺胺甲恶唑 (SMX)。实验表明PC/PS、AC/PS 和 CNT/PS 中 SMX 的去除依赖于 pH 值,动力学遵循 pH 4 > pH 7 > pH 9 的顺序。溶液 pH 同时影响 SMX 的吸附和降解,但后者更深刻。化学猝灭实验、电化学测量、动力学计算和 ATR-FTIR 测试共同表明,高 pH 不利于自由基和非自由基氧化。在 PC/PS 体系中,增加 pH 值会降低 PC 表面酚类 -OH 的量,从而抑制 SO 的生成4 •-和• OH 由于缺乏电子供体。对于 AC/PS 体系,升高的 pH 值阻碍了 AC 和 PS 之间的相互作用,从而抑制了表面结合自由基的形成。CNT/PS 启动了电子转移途径,增加 pH 值会降低表面 CNT-PS* 复合物的氧化电位,这不利于吸附污染物的非自由基氧化。因此,这项工作的结果将推进目前对含碳材料催化的 PS-AOP 中 pH 值的内在影响的认识,以用于废水净化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号