Water Research ( IF 11.4 ) Pub Date : 2022-09-14 , DOI: 10.1016/j.watres.2022.119120 Qingqing Kong 1 , Yanheng Pan 1 , Xin Lei 1 , Yangjian Zhou 1 , Yu Lei 1 , Jianglin Peng 1 , Xinran Zhang 1 , Ran Yin 2 , Chii Shang 2 , Xin Yang 1
|
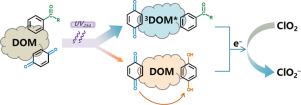
The triplet states of dissolved organic matter (3DOM*) have been well known to oxidize various organic contaminants, but evidence of their reducing properties are largely scarce. In this work, chlorine dioxide (ClO2) as a single-electron oxidant was used as a probe to evaluate the reduction property of 3DOM*. The reduction of ClO2 to chlorite was observed in the solutions of model photosensitizers (i.e., 4-carboxybenzophenone, benzophenone, acetophenone, 3-methoxyacetophenone, naphthalene, and xanthone) during UV irradiation with the presence of ClO2, though they are resistant to ClO2 oxidation in the dark. The reducing property of the triplet states of photosensitizers was verified and their second-order reaction rate constants with ClO2 were determined to be in the range of 1.45(± 0.03)× 109 – 2.18(± 0.06) × 109 M−1 s−1 at pH 7.0. The quenching tests excluded the role of other reactive species (e.g., HO•, O(3P), Cl•, ClO• and HOCl/OCl–, O2•– and eaq–) in ClO2 reduction to chlorite when using model photosensitizers and DOM isolates. Chlorite formation was 48.1–90.4% and 4812.8–7721.8% higher during UV irradiation with the presence of ClO2 and DOM than those without UV irradiation or without DOM present, respectively. The enhancement was attributed to the enhanced electron donating capacity (chlorite precursors) of DOM upon UV irradiation and also to 3DOM* acting as an electron donor reducing ClO2 to chlorite. This study highlighted the important role of 3DOM* as a reductant.
中文翻译:

通过从二氧化氯到亚氯酸盐的转变探索三重态有机物 (3DOM*) 的还原特性
众所周知,溶解有机物的三重态 ( 3 DOM*) 会氧化各种有机污染物,但其还原特性的证据却很少。在这项工作中,二氧化氯(ClO 2)作为单电子氧化剂被用作探针来评估3 DOM* 的还原性能。在存在 ClO 2的 UV 照射期间,在模型光敏剂(即 4-羧基二苯甲酮、二苯甲酮、苯乙酮、3-甲氧基苯乙酮、萘和呫吨酮)溶液中观察到ClO 2还原为亚氯酸盐,尽管它们对二氧化氯在黑暗中氧化。验证了光敏剂三重态的还原性,确定其与ClO 2的二级反应速率常数在1.45(±0.03)×10 9 – 2.18(±0.06)×10 9 M -1范围内s -1在 pH 7.0 时。淬火测试排除了 ClO 2中其他活性物质(例如,HO •、O( 3 P)、Cl •、ClO •和 HOCl/OCl –、O 2 •–和 e aq –)的作用使用模型光敏剂和 DOM 分离物时还原为亚氯酸盐。在存在 ClO 2和 DOM 的情况下,在紫外线照射期间,绿泥石的形成率分别比没有紫外线照射或不存在 DOM的情况下高 48.1-90.4% 和 4812.8-7721.8% 。这种增强归因于 DOM 在紫外线照射下的电子供体能力(亚氯酸盐前体)增强,也归因于3 DOM* 作为电子供体将 ClO 2还原为亚氯酸盐。本研究强调了3 DOM* 作为还原剂的重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号