当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nano-scale investigation of organic C sequestration and distribution on Fe oxides during ferrihydrite transformation: effect of Al-substitution
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2022-09-14 , DOI: 10.1039/d2en00505k Fu Liu 1 , Zecong Ding 1 , Yang Lu 2 , Rong Li 1 , Zhenqing Shi 1
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2022-09-14 , DOI: 10.1039/d2en00505k Fu Liu 1 , Zecong Ding 1 , Yang Lu 2 , Rong Li 1 , Zhenqing Shi 1
Affiliation
|
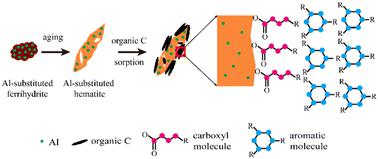
Iron (Fe) oxides are known to strongly interact with organic matter and play a vital role in soil organic carbon (C) stabilization. Poorly crystalline Fe oxides, such as ferrihydrite, can undergo a transformation process into more crystalline minerals, which is often accompanied by the presence of foreign elements, e.g., aluminum (Al), in natural environments. However, it is still unclear how the presence of Al affects the retention and the spatial distribution of organic C on Fe oxides during the Fe oxide transformation process. In this study, the sorption capacity of Al-substituted Fe oxides for organic C was quantified and the spatial distribution of organic C species was visualized at different aging times of Fe oxides. Spherical aberration corrected scanning transmission electron microscopy (Cs-STEM) and X-ray diffraction (XRD) results indicated that the Al-substitution slowed down the ferrihydrite transformation rate and changed the transformation products from rhombus-shape hematite into shuttle- and disk-shape. Wet chemistry experiments showed that Al-substitution in ferrihydrite increased the sorption capacity of organic C during the transformation process. Electron energy loss spectroscopy (EELS) results demonstrated that the carboxyl C was preferentially sorbed onto the surfaces of Fe oxides, forming the inner layer of the organo–mineral interfaces, while the aromatic C was dominantly bound to the outer layer of the organo–mineral interfaces, which supported the layer-by-layer “onion” model of organic C accumulation on minerals. Overall, our results help to elucidate the mechanisms of organic C sequestration during the Fe oxide transformation process in the presence of Al, which would be helpful for predicting organic C cycling in natural environments.
中文翻译:

水铁矿转化过程中 Fe 氧化物上有机 C 螯合和分布的纳米级研究:Al 取代的影响
众所周知,铁 (Fe) 氧化物会与有机物发生强烈相互作用,并在土壤有机碳 (C) 稳定中发挥重要作用。结晶度差的氧化铁,如水铁矿,可以经历转变为结晶度更高的矿物,这通常伴随着外来元素的存在,例如, 铝 (Al), 在自然环境中。然而,在氧化铁转化过程中,铝的存在如何影响有机碳在氧化铁上的保留和空间分布仍不清楚。在这项研究中,量化了 Al 取代的 Fe 氧化物对有机 C 的吸附能力,并在 Fe 氧化物的不同老化时间可视化了有机 C 物种的空间分布。球面像差校正扫描透射电子显微镜(Cs-STEM)和X射线衍射(XRD)结果表明,Al取代减缓了水铁矿的转变速率,并将转变产物从菱形赤铁矿转变为梭形和盘形。 . 湿化学实验表明,水铁矿中的铝取代增加了转化过程中有机碳的吸附能力。电子能量损失谱 (EELS) 结果表明,羧基 C 优先吸附在 Fe 氧化物的表面,形成有机-矿物界面的内层,而芳香族 C 主要结合到有机-矿物的外层。界面,支持矿物有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。电子能量损失谱 (EELS) 结果表明,羧基 C 优先吸附在 Fe 氧化物的表面,形成有机-矿物界面的内层,而芳香族 C 主要结合到有机-矿物的外层。界面,支持矿物有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。电子能量损失谱 (EELS) 结果表明,羧基 C 优先吸附在 Fe 氧化物的表面,形成有机-矿物界面的内层,而芳香族 C 主要结合到有机-矿物的外层。界面,支持矿物有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。这支持了矿物上有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。这支持了矿物上有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。
更新日期:2022-09-14
中文翻译:

水铁矿转化过程中 Fe 氧化物上有机 C 螯合和分布的纳米级研究:Al 取代的影响
众所周知,铁 (Fe) 氧化物会与有机物发生强烈相互作用,并在土壤有机碳 (C) 稳定中发挥重要作用。结晶度差的氧化铁,如水铁矿,可以经历转变为结晶度更高的矿物,这通常伴随着外来元素的存在,例如, 铝 (Al), 在自然环境中。然而,在氧化铁转化过程中,铝的存在如何影响有机碳在氧化铁上的保留和空间分布仍不清楚。在这项研究中,量化了 Al 取代的 Fe 氧化物对有机 C 的吸附能力,并在 Fe 氧化物的不同老化时间可视化了有机 C 物种的空间分布。球面像差校正扫描透射电子显微镜(Cs-STEM)和X射线衍射(XRD)结果表明,Al取代减缓了水铁矿的转变速率,并将转变产物从菱形赤铁矿转变为梭形和盘形。 . 湿化学实验表明,水铁矿中的铝取代增加了转化过程中有机碳的吸附能力。电子能量损失谱 (EELS) 结果表明,羧基 C 优先吸附在 Fe 氧化物的表面,形成有机-矿物界面的内层,而芳香族 C 主要结合到有机-矿物的外层。界面,支持矿物有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。电子能量损失谱 (EELS) 结果表明,羧基 C 优先吸附在 Fe 氧化物的表面,形成有机-矿物界面的内层,而芳香族 C 主要结合到有机-矿物的外层。界面,支持矿物有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。电子能量损失谱 (EELS) 结果表明,羧基 C 优先吸附在 Fe 氧化物的表面,形成有机-矿物界面的内层,而芳香族 C 主要结合到有机-矿物的外层。界面,支持矿物有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。这支持了矿物上有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。这支持了矿物上有机碳积累的逐层“洋葱”模型。总体而言,我们的结果有助于阐明在 Al 存在下氧化铁转化过程中有机 C 螯合的机制,这将有助于预测自然环境中的有机 C 循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号