当前位置:
X-MOL 学术
›
Adv. Therap.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cell-Free, Dendritic Cell-Mimicking Extracellular Blebs for Molecularly Controlled Vaccination
Advanced Therapeutics ( IF 3.7 ) Pub Date : 2022-09-13 , DOI: 10.1002/adtp.202200125 Melissa N Thone 1, 2 , Jee Young Chung 2 , Dominique Ingato 1 , Margaret L Lugin 1 , Young Jik Kwon 1, 2, 3, 4
Advanced Therapeutics ( IF 3.7 ) Pub Date : 2022-09-13 , DOI: 10.1002/adtp.202200125 Melissa N Thone 1, 2 , Jee Young Chung 2 , Dominique Ingato 1 , Margaret L Lugin 1 , Young Jik Kwon 1, 2, 3, 4
Affiliation
|
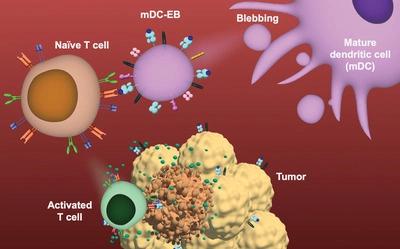
Dendritic cells (DCs) are prime targets for vaccination and immunotherapy. However, limited control over antigen presentation at a desired maturation status in these plastic materials remains a fundamental challenge in efficiently orchestrating a controlled immune response. DC-derived extracellular vesicles (EVs) can overcome some of these issues, but have significant production challenges. Herein, this work employs a unique chemicallyinduced method for production of DC-derived extracellular blebs (DC-EBs) that overcome the barriers of DC and DC-derived EV vaccines. DC-EBs are molecular snapshots of DCs in time, cell-like particles with fixed stimulatory profiles for controlled immune signaling. DC-EBs are produced in an order of magnitude more quickly and efficiently than conventional EVs and display stable structural integrity and antigen presentation compared to live DCs. Multi-omic analysis confirmed DC-EBs are majorly pure plasma membrane vesicles that are homogeneous at the single-vesicle level, critical for safe and effective vaccination. Immature versus mature molecular profiles on DC-EBs exhibit molecularly modulated immune responses compared to live DCs, improving remission and survival of tumor-challenged mice via generation of antigen-specific T cells. For the first time, DC-EBs make their case for use in vaccines and for their potential in modulating other immune responses, potentially in combination with other immunotherapeutics.
中文翻译:

用于分子控制疫苗接种的无细胞、模仿树突状细胞的细胞外泡
树突状细胞(DC)是疫苗接种和免疫治疗的主要目标。然而,在这些塑料材料中,在所需的成熟状态下对抗原呈递的有限控制仍然是有效协调受控免疫反应的基本挑战。 DC 衍生的细胞外囊泡 (EV) 可以克服其中一些问题,但存在重大的生产挑战。在此,这项工作采用了一种独特的化学诱导方法来生产 DC 衍生的细胞外泡 (DC-EB),克服了 DC 和 DC 衍生的 EV 疫苗的障碍。 DC-EB 是 DC 及时的分子快照,是具有固定刺激曲线的细胞样颗粒,用于控制免疫信号。 DC-EB 的生产速度比传统 EV 快一个数量级,效率也高一个数量级,并且与活 DC 相比,它表现出稳定的结构完整性和抗原呈递。多组学分析证实 DC-EB 主要是纯质膜囊泡,在单囊泡水平上是均质的,这对于安全有效的疫苗接种至关重要。与活 DC 相比,DC-EB 上的未成熟分子与成熟分子谱表现出分子调节的免疫反应,通过生成抗原特异性 T 细胞改善肿瘤小鼠的缓解和存活。 DC-EB 首次证明其在疫苗中的应用及其在调节其他免疫反应方面的潜力,并有可能与其他免疫治疗药物结合使用。
更新日期:2022-09-13
中文翻译:

用于分子控制疫苗接种的无细胞、模仿树突状细胞的细胞外泡
树突状细胞(DC)是疫苗接种和免疫治疗的主要目标。然而,在这些塑料材料中,在所需的成熟状态下对抗原呈递的有限控制仍然是有效协调受控免疫反应的基本挑战。 DC 衍生的细胞外囊泡 (EV) 可以克服其中一些问题,但存在重大的生产挑战。在此,这项工作采用了一种独特的化学诱导方法来生产 DC 衍生的细胞外泡 (DC-EB),克服了 DC 和 DC 衍生的 EV 疫苗的障碍。 DC-EB 是 DC 及时的分子快照,是具有固定刺激曲线的细胞样颗粒,用于控制免疫信号。 DC-EB 的生产速度比传统 EV 快一个数量级,效率也高一个数量级,并且与活 DC 相比,它表现出稳定的结构完整性和抗原呈递。多组学分析证实 DC-EB 主要是纯质膜囊泡,在单囊泡水平上是均质的,这对于安全有效的疫苗接种至关重要。与活 DC 相比,DC-EB 上的未成熟分子与成熟分子谱表现出分子调节的免疫反应,通过生成抗原特异性 T 细胞改善肿瘤小鼠的缓解和存活。 DC-EB 首次证明其在疫苗中的应用及其在调节其他免疫反应方面的潜力,并有可能与其他免疫治疗药物结合使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号