当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Antiviral Activity of the Thiazole Positional Isomers of a Potent HIV-1 Entry Inhibitor NBD-14270
ChemMedChem ( IF 3.6 ) Pub Date : 2022-09-12 , DOI: 10.1002/cmdc.202200344 Alexander V Kurkin 1 , Francesca Curreli 2 , Ildar R Iusupov 1 , Evgeniy A Spiridonov 1 , Shahad Ahmed 2 , Pavel O Markov 1 , Ekaterina V Manasova 1 , Andrea Altieri 1 , Asim K Debnath 2
ChemMedChem ( IF 3.6 ) Pub Date : 2022-09-12 , DOI: 10.1002/cmdc.202200344 Alexander V Kurkin 1 , Francesca Curreli 2 , Ildar R Iusupov 1 , Evgeniy A Spiridonov 1 , Shahad Ahmed 2 , Pavel O Markov 1 , Ekaterina V Manasova 1 , Andrea Altieri 1 , Asim K Debnath 2
Affiliation
|
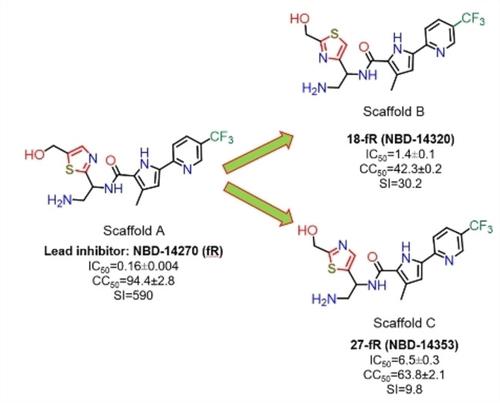
The primary goal of this work was to determine whether positional isomers of the thiazole ring in one of the lead inhibitors, NBD-14270, may lead to an improvement in antiviral potency and cytotoxicity, and to derive SAR information. Based on how the thiazole ring (Scaffold A) is bound to NBD-14270, only two other positional isomers are possible: Scaffolds B and C. Antiviral assay data show that Scaffold A provides the best potency and a high selectivity index (SI).
中文翻译:

一种有效的 HIV-1 进入抑制剂 NBD-14270 的噻唑位置异构体的设计、合成和抗病毒活性
这项工作的主要目标是确定其中一种先导抑制剂 NBD-14270 中噻唑环的位置异构体是否可能导致抗病毒效力和细胞毒性的改善,并获得 SAR 信息。根据噻唑环(Scaffold A)与 NBD-14270 的结合方式,只有两种其他位置异构体是可能的:Scaffolds B 和 C。抗病毒测定数据显示 Scaffold A 提供最佳效力和高选择性指数 (SI)。
更新日期:2022-09-12
中文翻译:

一种有效的 HIV-1 进入抑制剂 NBD-14270 的噻唑位置异构体的设计、合成和抗病毒活性
这项工作的主要目标是确定其中一种先导抑制剂 NBD-14270 中噻唑环的位置异构体是否可能导致抗病毒效力和细胞毒性的改善,并获得 SAR 信息。根据噻唑环(Scaffold A)与 NBD-14270 的结合方式,只有两种其他位置异构体是可能的:Scaffolds B 和 C。抗病毒测定数据显示 Scaffold A 提供最佳效力和高选择性指数 (SI)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号