当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Study and Thermodynamic Modeling of Mesalamine and Azathioprine Solubility in Some Choline Chloride-Based Deep Eutectic Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-12 , DOI: 10.1021/acs.jced.2c00408 Tahereh Amini 1 , Ali Haghtalab 1 , Jaber Yousefi Seyf 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-12 , DOI: 10.1021/acs.jced.2c00408 Tahereh Amini 1 , Ali Haghtalab 1 , Jaber Yousefi Seyf 2
Affiliation
|
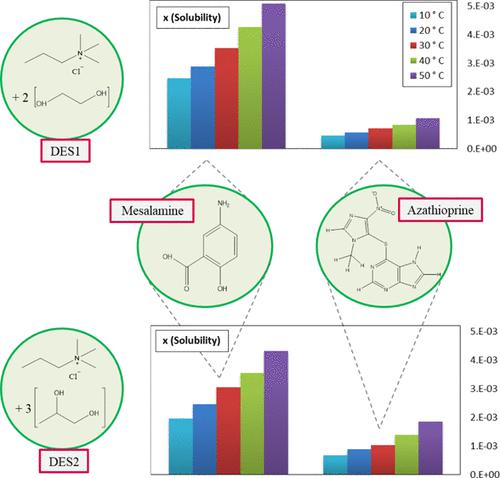
The present study attempted to measure the solubility of mesalamine and azathioprine in the aqueous solution of some deep eutectic solvents (DESs) at various DES weight fractions (0–1) over the temperature range of 283.15–323.15 K. We prepared the DESs by combining choline chloride (ChCl, hydrogen bond acceptor, HBA) and ethylene glycol (EG) and 1,2-propanediol (PD) (hydrogen bond donors, HBDs) with molar ratios of 1:2 and 1:3, respectively. The results indicate that the solubility is significantly increased up to 30-fold for mesalamine and 75-fold for azathioprine compared to water. Besides, the solubility of both pharmaceuticals is enhanced by increasing the temperature and weight fraction of DESs. Also, DES1 (ChCl + EG) shows higher solubility for mesalamine than DES2 (ChCl + PD), which is the opposite for azathioprine. The experimental solubility data were accurately correlated using the local composition models such as Wilson, nonrandom two-liquid theory (NRTL), and universal quasi-chemical (UNIQUAC). On the other hand, we applied the nonrandom two-liquid-segment activity coefficient (NRTL-SAC) and universal quasi-chemical segment activity coefficient (UNIQUAC-SAC) models to correlate and predict the solubility of the mesalamine and azathioprine in DES1 and DES2. In addition, the calculated values of the thermodynamic functions of dissolution reveal that in the dissolution process of both drugs, enthalpy is the primary contribution term to the standard Gibbs free energy.
中文翻译:

美沙拉嗪和硫唑嘌呤在一些氯化胆碱基低共熔溶剂中溶解度的实验研究和热力学模型
本研究试图在 283.15-323.15 K 的温度范围内测量不同 DES 重量分数 (0-1) 的一些深共熔溶剂 (DES) 水溶液中美沙拉嗪和硫唑嘌呤的溶解度。我们通过结合制备 DES氯化胆碱(ChCl,氢键受体,HBA)和乙二醇(EG)和1,2-丙二醇(PD)(氢键供体,HBD),摩尔比分别为1:2和1:3。结果表明,与水相比,美沙拉嗪的溶解度显着增加了 30 倍,硫唑嘌呤的溶解度增加了 75 倍。此外,通过提高 DES 的温度和重量分数,两种药物的溶解度都得到了增强。此外,DES1 (ChCl + EG) 对美沙拉嗪的溶解度高于 DES2 (ChCl + PD),这与硫唑嘌呤相反。使用局部成分模型(例如 Wilson、非随机二液理论 (NRTL) 和通用准化学 (UNIQUAC))准确关联实验溶解度数据。另一方面,我们应用非随机二液链段活性系数 (NRTL-SAC) 和通用准化学链段活性系数 (UNIQUAC-SAC) 模型来关联和预测美沙拉嗪和硫唑嘌呤在 DES1 和 DES2 中的溶解度. 此外,溶出热力学函数的计算值表明,在两种药物的溶出过程中,焓是对标准吉布斯自由能的主要贡献项。我们应用非随机二液链段活性系数 (NRTL-SAC) 和通用准化学链段活性系数 (UNIQUAC-SAC) 模型来关联和预测美沙拉嗪和硫唑嘌呤在 DES1 和 DES2 中的溶解度。此外,溶出热力学函数的计算值表明,在两种药物的溶出过程中,焓是对标准吉布斯自由能的主要贡献项。我们应用非随机二液链段活性系数 (NRTL-SAC) 和通用准化学链段活性系数 (UNIQUAC-SAC) 模型来关联和预测美沙拉嗪和硫唑嘌呤在 DES1 和 DES2 中的溶解度。此外,溶出热力学函数的计算值表明,在两种药物的溶出过程中,焓是对标准吉布斯自由能的主要贡献项。
更新日期:2022-09-12
中文翻译:

美沙拉嗪和硫唑嘌呤在一些氯化胆碱基低共熔溶剂中溶解度的实验研究和热力学模型
本研究试图在 283.15-323.15 K 的温度范围内测量不同 DES 重量分数 (0-1) 的一些深共熔溶剂 (DES) 水溶液中美沙拉嗪和硫唑嘌呤的溶解度。我们通过结合制备 DES氯化胆碱(ChCl,氢键受体,HBA)和乙二醇(EG)和1,2-丙二醇(PD)(氢键供体,HBD),摩尔比分别为1:2和1:3。结果表明,与水相比,美沙拉嗪的溶解度显着增加了 30 倍,硫唑嘌呤的溶解度增加了 75 倍。此外,通过提高 DES 的温度和重量分数,两种药物的溶解度都得到了增强。此外,DES1 (ChCl + EG) 对美沙拉嗪的溶解度高于 DES2 (ChCl + PD),这与硫唑嘌呤相反。使用局部成分模型(例如 Wilson、非随机二液理论 (NRTL) 和通用准化学 (UNIQUAC))准确关联实验溶解度数据。另一方面,我们应用非随机二液链段活性系数 (NRTL-SAC) 和通用准化学链段活性系数 (UNIQUAC-SAC) 模型来关联和预测美沙拉嗪和硫唑嘌呤在 DES1 和 DES2 中的溶解度. 此外,溶出热力学函数的计算值表明,在两种药物的溶出过程中,焓是对标准吉布斯自由能的主要贡献项。我们应用非随机二液链段活性系数 (NRTL-SAC) 和通用准化学链段活性系数 (UNIQUAC-SAC) 模型来关联和预测美沙拉嗪和硫唑嘌呤在 DES1 和 DES2 中的溶解度。此外,溶出热力学函数的计算值表明,在两种药物的溶出过程中,焓是对标准吉布斯自由能的主要贡献项。我们应用非随机二液链段活性系数 (NRTL-SAC) 和通用准化学链段活性系数 (UNIQUAC-SAC) 模型来关联和预测美沙拉嗪和硫唑嘌呤在 DES1 和 DES2 中的溶解度。此外,溶出热力学函数的计算值表明,在两种药物的溶出过程中,焓是对标准吉布斯自由能的主要贡献项。









































 京公网安备 11010802027423号
京公网安备 11010802027423号