当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-in-human study of ONO-4578, an antagonist of prostaglandin E2 receptor 4, alone and with nivolumab in solid tumors
Cancer Science ( IF 4.5 ) Pub Date : 2022-09-09 , DOI: 10.1111/cas.15574 Satoru Iwasa 1 , Takafumi Koyama 1 , Makoto Nishino 1 , Shunsuke Kondo 1 , Kazuki Sudo 1 , Kan Yonemori 1, 2 , Tatsuya Yoshida 1, 3 , Kenji Tamura 4 , Toshio Shimizu 1 , Yutaka Fujiwara 1 , Shigehisa Kitano 1 , Akihiko Shimomura 1 , Jun Sato 1 , Fumiharu Yokoyama 5 , Hiroyuki Iida 6 , Maki Kondo 7 , Noboru Yamamoto 1
Cancer Science ( IF 4.5 ) Pub Date : 2022-09-09 , DOI: 10.1111/cas.15574 Satoru Iwasa 1 , Takafumi Koyama 1 , Makoto Nishino 1 , Shunsuke Kondo 1 , Kazuki Sudo 1 , Kan Yonemori 1, 2 , Tatsuya Yoshida 1, 3 , Kenji Tamura 4 , Toshio Shimizu 1 , Yutaka Fujiwara 1 , Shigehisa Kitano 1 , Akihiko Shimomura 1 , Jun Sato 1 , Fumiharu Yokoyama 5 , Hiroyuki Iida 6 , Maki Kondo 7 , Noboru Yamamoto 1
Affiliation
|
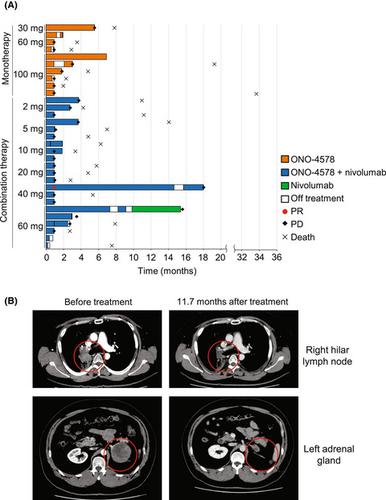
EP4, a prostaglandin E2 receptor, has shown an immunosuppressive activity on cancer cells. This first-in-human study evaluated ONO-4578, a highly selective EP4 antagonist, as monotherapy and in combination with nivolumab in patients with advanced or metastatic solid tumors. A daily dose ranging from 30 mg to 100 mg of ONO-4578 monotherapy and that ranging from 2 mg to 60 mg of ONO-4578 with biweekly nivolumab 240 mg were administered. A total of 31 patients were enrolled, 10 receiving monotherapy and 21 receiving combination therapy. Overall, 26 patients experienced treatment-related adverse events. Dose-limiting toxicities were observed in three patients; one of six patients receiving 100 mg monotherapy developed grade 3 duodenal ulcer and two of six patients receiving 60 mg combination therapy developed either grade 3 erythema multiforme or grade 3 increased amylase and grade 4 increased lipase. One patient with small-cell lung cancer who received 40 mg combination therapy had a partial response, and three patients with monotherapy and six patients with combination therapy had stable disease. Pharmacodynamics analyses showed that ONO-4578 had EP4 antagonistic activity at doses as low as 2 mg. In conclusion, the maximum tolerated dose of ONO-4578 alone or in combination with nivolumab was not reached. ONO-4578 was well tolerated at the tested doses and showed signs of antitumor activity. Considering safety, efficacy, and pharmacokinetics/pharmacodynamics results, ONO-4578 40 mg daily with nivolumab 240 mg biweekly was selected as the recommended dose for future clinical trials. (Registration: JapicCTI-173,496 and NCT03155061).
中文翻译:

ONO-4578 的首次人体研究,一种前列腺素 E2 受体 4 的拮抗剂,单独使用和与 nivolumab 一起用于治疗实体瘤
EP4,一种前列腺素 E 2受体,已显示出对癌细胞的免疫抑制活性。这项首次人体研究评估了 ONO-4578(一种高选择性 EP4 拮抗剂)作为单一疗法以及与 nivolumab 联合用于晚期或转移性实体瘤患者的疗效。每日剂量范围为 30 mg 至 100 mg 的 ONO-4578 单一疗法和范围为 2 mg 至 60 mg 的 ONO-4578 以及每两周一次的 nivolumab 240 mg。共有 31 名患者入组,其中 10 名接受单一疗法,21 名接受联合疗法。总体而言,26 名患者经历了与治疗相关的不良事件。在三名患者中观察到剂量限制性毒性;接受 100 mg 单药治疗的 6 名患者中有 1 名发展为 3 级十二指肠溃疡,接受 60 mg 联合治疗的 6 名患者中有 2 名发展为 3 级多形性红斑或 3 级淀粉酶升高和 4 级脂肪酶升高。接受 40 mg 联合治疗的 1 名小细胞肺癌患者出现部分反应,3 名接受单药治疗的患者和 6 名接受联合治疗的患者病情稳定。药效学分析表明,ONO-4578 在低至 2 mg 的剂量下具有 EP4 拮抗活性。总之,未达到 ONO-4578 单独或与 nivolumab 联合使用的最大耐受剂量。ONO-4578 在测试剂量下耐受性良好,并显示出抗肿瘤活性迹象。考虑到安全性、有效性和药代动力学/药效学结果,ONO-4578 每天 40 mg 和 nivolumab 240 mg 每两周被选为未来临床试验的推荐剂量。(注册号:JapicCTI-173,496 和 NCT03155061)。
更新日期:2022-09-09
中文翻译:

ONO-4578 的首次人体研究,一种前列腺素 E2 受体 4 的拮抗剂,单独使用和与 nivolumab 一起用于治疗实体瘤
EP4,一种前列腺素 E 2受体,已显示出对癌细胞的免疫抑制活性。这项首次人体研究评估了 ONO-4578(一种高选择性 EP4 拮抗剂)作为单一疗法以及与 nivolumab 联合用于晚期或转移性实体瘤患者的疗效。每日剂量范围为 30 mg 至 100 mg 的 ONO-4578 单一疗法和范围为 2 mg 至 60 mg 的 ONO-4578 以及每两周一次的 nivolumab 240 mg。共有 31 名患者入组,其中 10 名接受单一疗法,21 名接受联合疗法。总体而言,26 名患者经历了与治疗相关的不良事件。在三名患者中观察到剂量限制性毒性;接受 100 mg 单药治疗的 6 名患者中有 1 名发展为 3 级十二指肠溃疡,接受 60 mg 联合治疗的 6 名患者中有 2 名发展为 3 级多形性红斑或 3 级淀粉酶升高和 4 级脂肪酶升高。接受 40 mg 联合治疗的 1 名小细胞肺癌患者出现部分反应,3 名接受单药治疗的患者和 6 名接受联合治疗的患者病情稳定。药效学分析表明,ONO-4578 在低至 2 mg 的剂量下具有 EP4 拮抗活性。总之,未达到 ONO-4578 单独或与 nivolumab 联合使用的最大耐受剂量。ONO-4578 在测试剂量下耐受性良好,并显示出抗肿瘤活性迹象。考虑到安全性、有效性和药代动力学/药效学结果,ONO-4578 每天 40 mg 和 nivolumab 240 mg 每两周被选为未来临床试验的推荐剂量。(注册号:JapicCTI-173,496 和 NCT03155061)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号