Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-09-09 , DOI: 10.1016/j.bmcl.2022.128980 David P Stockdale 1 , John A Beutler 2 , David F Wiemer 1
|
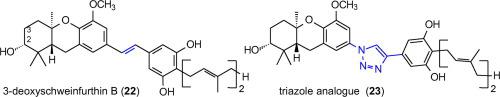
The stilbene moiety is commonly found in natural products and these compounds display an extraordinary range of biological activity. Efforts to derive useful drugs from stilbenes must address the potential liabilities of this structure, including a propensity for cis/trans isomerization. To identify olefin replacements that address this limitation while preserving biological activity we have prepared analogues of two bioactive stilbenes, a pawhuskin and a schweinfurthin, where a 1,2,3-triazole ring formally replaces the stilbene double bond. The new schweinfurthin analogue (23) has been tested for anti-proliferative activity against 60 cell lines, and shows a strong correlation of bioactivity when compared to the compound that inspired its synthesis (22).
中文翻译:

三唑取代生物活性二苯乙烯中的中心烯烃
芪部分常见于天然产物中,这些化合物显示出非凡的生物活性。从二苯乙烯中提取有用药物的努力必须解决这种结构的潜在问题,包括顺式/反式异构化的倾向。为了确定在保持生物活性的同时解决这一限制的烯烃替代物,我们制备了两种生物活性二苯乙烯的类似物,一种爪胡斯金和一种施韦因富辛,其中 1,2,3-三唑环正式取代了二苯乙烯双键。新的 schweinfurthin 类似物 ( 23 ) 已针对 60 种细胞系进行了抗增殖活性测试,与激发其合成的化合物 ( 22 ) 相比,它显示出强烈的生物活性相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号