当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Measurements for Equilibrium Conditions of Carbon Dioxide Hydrate Mixtures with Each Additive of 1,3-Dioxane, Acetamide, Cyclopentanol, Cyclopentanone, or 1,3,5-Trioxane
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-08 , DOI: 10.1021/acs.jced.2c00339 Muoi Tang, Hsin-Wei Wang, Yi-Ping Wu, Jung-Chin Tsai, Yan-Ping Chen
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-08 , DOI: 10.1021/acs.jced.2c00339 Muoi Tang, Hsin-Wei Wang, Yi-Ping Wu, Jung-Chin Tsai, Yan-Ping Chen
|
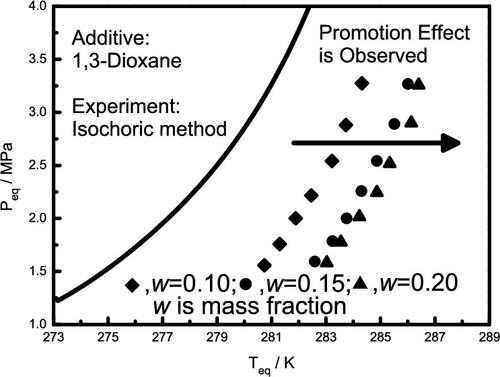
Gas hydrate research studies have received much attention in recent years. In addition to the availability of naturally existing methane hydrates as a new energy source, carbon dioxide hydrates also offer the potential for carbon capture and sequestration. This study reports the equilibrium temperatures and pressures at the dissociation points where three phases of hydrate (H), liquid water (Lw), and vapor (V) coexisted for carbon dioxide hydrates with various additives. The equilibrium conditions for carbon dioxide with pure water and each additive of 1,3-dioxane (0.10, 0.15, and 0.20 mass fractions), acetamide (0.10 and 0.20 mass fractions), cyclopentanol (0.08 mass fraction), cyclopentanone (0.05 mass fraction), or 1,3,5-trioxane (0.15 mass fraction) were experimentally measured in the pressure range of 1.5–3.3 MPa using the isochoric method. Additives of 1,3-dioxane, cyclopentanol, cyclopentanone, or 1,3,5-trioxane presented a promotion effect on the formation of carbon dioxide hydrates. The maximum promotion result under isobaric conditions was 7.8 K with the addition of 0.20 mass fraction of 1,3-dioxane. The addition of acetamide showed an inhibition effect, with a maximum inhibition result of 5 K at 0.20 mass fraction. Comparisons of the promotion or inhibition effect for various additives are illustrated. To simulate seawater conditions, equilibrium conditions for carbon dioxide with brine and each additive of acetamide (0.10 and 0.20 mass fractions) or 1,3-dioxane (0.20 mass fraction) were also reported. This study employed the Clausius–Clapeyron equation to estimate possible hydrate structures for systems with various additives. The estimated hydrate structures were in good agreement with the promotion or inhibition effect of carbon dioxide hydrate mixtures with various additives.
中文翻译:

具有 1,3-二恶烷、乙酰胺、环戊醇、环戊酮或 1,3,5-三恶烷的每种添加剂的二氧化碳水合物混合物平衡条件的实验测量
近年来,天然气水合物研究备受关注。除了天然存在的甲烷水合物作为新能源的可用性外,二氧化碳水合物还提供了碳捕获和封存的潜力。本研究报告了解离点的平衡温度和压力,其中水合物 (H)、液态水 (L w) 和蒸气 (V) 共存的二氧化碳水合物与各种添加剂。二氧化碳与纯水和1,3-二恶烷(0.10、0.15和0.20质量分数)、乙酰胺(0.10和0.20质量分数)、环戊醇(0.08质量分数)、环戊酮(0.05质量分数)的平衡条件) 或 1,3,5-三恶烷 (0.15 质量分数) 使用等容法在 1.5-3.3 MPa 的压力范围内进行了实验测量。1,3-二恶烷、环戊醇、环戊酮或1,3,5-三恶烷的添加剂对二氧化碳水合物的形成有促进作用。添加0.20质量分数的1,3-二恶烷在等压条件下的最大促进结果为7.8 K。添加乙酰胺显示出抑制作用,在0.20质量分数时最大抑制结果为5 K。说明了各种添加剂的促进或抑制效果的比较。为了模拟海水条件,还报告了二氧化碳与盐水和乙酰胺(0.10 和 0.20 质量分数)或 1,3-二恶烷(0.20 质量分数)的每种添加剂的平衡条件。本研究采用克劳修斯-克拉佩龙方程来估计具有各种添加剂的系统可能的水合物结构。估计的水合物结构与具有各种添加剂的二氧化碳水合物混合物的促进或抑制作用非常吻合。本研究采用克劳修斯-克拉佩龙方程来估计具有各种添加剂的系统可能的水合物结构。估计的水合物结构与具有各种添加剂的二氧化碳水合物混合物的促进或抑制作用非常吻合。本研究采用克劳修斯-克拉佩龙方程来估计具有各种添加剂的系统可能的水合物结构。估计的水合物结构与具有各种添加剂的二氧化碳水合物混合物的促进或抑制作用非常吻合。
更新日期:2022-09-08
中文翻译:

具有 1,3-二恶烷、乙酰胺、环戊醇、环戊酮或 1,3,5-三恶烷的每种添加剂的二氧化碳水合物混合物平衡条件的实验测量
近年来,天然气水合物研究备受关注。除了天然存在的甲烷水合物作为新能源的可用性外,二氧化碳水合物还提供了碳捕获和封存的潜力。本研究报告了解离点的平衡温度和压力,其中水合物 (H)、液态水 (L w) 和蒸气 (V) 共存的二氧化碳水合物与各种添加剂。二氧化碳与纯水和1,3-二恶烷(0.10、0.15和0.20质量分数)、乙酰胺(0.10和0.20质量分数)、环戊醇(0.08质量分数)、环戊酮(0.05质量分数)的平衡条件) 或 1,3,5-三恶烷 (0.15 质量分数) 使用等容法在 1.5-3.3 MPa 的压力范围内进行了实验测量。1,3-二恶烷、环戊醇、环戊酮或1,3,5-三恶烷的添加剂对二氧化碳水合物的形成有促进作用。添加0.20质量分数的1,3-二恶烷在等压条件下的最大促进结果为7.8 K。添加乙酰胺显示出抑制作用,在0.20质量分数时最大抑制结果为5 K。说明了各种添加剂的促进或抑制效果的比较。为了模拟海水条件,还报告了二氧化碳与盐水和乙酰胺(0.10 和 0.20 质量分数)或 1,3-二恶烷(0.20 质量分数)的每种添加剂的平衡条件。本研究采用克劳修斯-克拉佩龙方程来估计具有各种添加剂的系统可能的水合物结构。估计的水合物结构与具有各种添加剂的二氧化碳水合物混合物的促进或抑制作用非常吻合。本研究采用克劳修斯-克拉佩龙方程来估计具有各种添加剂的系统可能的水合物结构。估计的水合物结构与具有各种添加剂的二氧化碳水合物混合物的促进或抑制作用非常吻合。本研究采用克劳修斯-克拉佩龙方程来估计具有各种添加剂的系统可能的水合物结构。估计的水合物结构与具有各种添加剂的二氧化碳水合物混合物的促进或抑制作用非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号