当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior and the Mechanism of HCl Gas in Four [EMIM]Cl-Based Deep Eutectic Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-08 , DOI: 10.1021/acs.jced.2c00357 Hui Shao 1, 2 , Jie Zhu 1, 2 , Lin Feng 1, 2 , Xin Liang 1, 3 , Yingzhou Lu 2 , Hong Meng 4 , Chunxi Li 1, 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-08 , DOI: 10.1021/acs.jced.2c00357 Hui Shao 1, 2 , Jie Zhu 1, 2 , Lin Feng 1, 2 , Xin Liang 1, 3 , Yingzhou Lu 2 , Hong Meng 4 , Chunxi Li 1, 2, 3
Affiliation
|
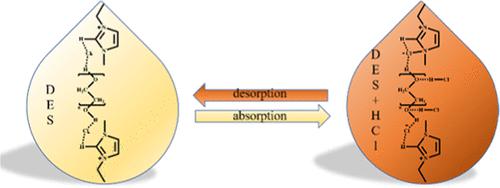
The solubility behavior of HCl gas in four 1-ethyl-3-methylimidazolium chloride([Emim]Cl)-based deep eutectic solvents (DESs), i.e., an [Emim]Cl mixture with 4-methylimidazole (DES1), 2-methylimidazole (DES2), malonic acid (DES3), and poly(ethylene glycol) 400 (DES4), was studied at 301.15–348.15 K and a 0–101.325 kPa partial pressure of HCl. The H-bonding interaction and the dissolution mechanism were studied via Fourier-transform infrared spectroscopy (FTIR), 1H NMR, and 13C NMR. The HCl solubility increases with the alkalinity of the DESs and its H-bonding interaction with the solvent and follows the order DES1 > DES2 > DES4 > DES3. The HCl solubility in DES1 is 0.584 g-HCl/g-DES with partially irreversible desorption due to its chemical interaction with HCl. In contrast, HCl is physically dissolved in DES3 and DES4 with facile and reversible desorption. The solution viscosity decreases sharply and the density increases slightly as the absorption proceeds. Considering its higher solubility, thermal stability, and facile desorption, DES4 is more viable for HCl absorption. The result is instructive for the development of a greener recovery process for HCl gas and the selection of efficient DESs.
中文翻译:

HCl气体在四种[EMIM]Cl基深共晶溶剂中的溶解行为和机理
HCl气体在四种1-乙基-3-甲基咪唑氯化物([Emim]Cl)基低共熔溶剂(DESs)中的溶解行为,即[Emim]Cl与4-甲基咪唑(DES1)、2-甲基咪唑的混合物(DES2)、丙二酸 (DES3) 和聚 (乙二醇) 400 (DES4) 在 301.15–348.15 K 和 0–101.325 kPa 的 HCl 分压下进行了研究。通过傅里叶变换红外光谱 (FTIR)、 1 H NMR 和13研究了 H 键相互作用和溶解机理C核磁共振。HCl 溶解度随着 DES 的碱度及其与溶剂的氢键相互作用而增加,并遵循 DES1 > DES2 > DES4 > DES3 的顺序。DES1 中的 HCl 溶解度为 0.584 g-HCl/g-DES,由于其与 HCl 的化学相互作用,具有部分不可逆的解吸。相比之下,HCl 以物理方式溶解在 DES3 和 DES4 中,解吸容易且可逆。随着吸收的进行,溶液粘度急剧下降,密度略有增加。考虑到其较高的溶解度、热稳定性和易于解吸,DES4 更适合 HCl 吸收。该结果对于开发更环保的 HCl 气体回收工艺和选择高效 DES 具有指导意义。
更新日期:2022-09-08
中文翻译:

HCl气体在四种[EMIM]Cl基深共晶溶剂中的溶解行为和机理
HCl气体在四种1-乙基-3-甲基咪唑氯化物([Emim]Cl)基低共熔溶剂(DESs)中的溶解行为,即[Emim]Cl与4-甲基咪唑(DES1)、2-甲基咪唑的混合物(DES2)、丙二酸 (DES3) 和聚 (乙二醇) 400 (DES4) 在 301.15–348.15 K 和 0–101.325 kPa 的 HCl 分压下进行了研究。通过傅里叶变换红外光谱 (FTIR)、 1 H NMR 和13研究了 H 键相互作用和溶解机理C核磁共振。HCl 溶解度随着 DES 的碱度及其与溶剂的氢键相互作用而增加,并遵循 DES1 > DES2 > DES4 > DES3 的顺序。DES1 中的 HCl 溶解度为 0.584 g-HCl/g-DES,由于其与 HCl 的化学相互作用,具有部分不可逆的解吸。相比之下,HCl 以物理方式溶解在 DES3 和 DES4 中,解吸容易且可逆。随着吸收的进行,溶液粘度急剧下降,密度略有增加。考虑到其较高的溶解度、热稳定性和易于解吸,DES4 更适合 HCl 吸收。该结果对于开发更环保的 HCl 气体回收工艺和选择高效 DES 具有指导意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号