当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaching High Stereoselectivity and Activity in Organocatalyzed Ring-Opening Polymerization of Racemic Lactide by the Combined Use of a Chiral (Thio)Urea and a N-Heterocyclic Carbene
ACS Macro Letters ( IF 5.1 ) Pub Date : 2022-09-06 , DOI: 10.1021/acsmacrolett.2c00457 Mohamed Samir Zaky 1 , Anne-Laure Wirotius 1 , Olivier Coulembier 2 , Gilles Guichard 3 , Daniel Taton 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2022-09-06 , DOI: 10.1021/acsmacrolett.2c00457 Mohamed Samir Zaky 1 , Anne-Laure Wirotius 1 , Olivier Coulembier 2 , Gilles Guichard 3 , Daniel Taton 1
Affiliation
|
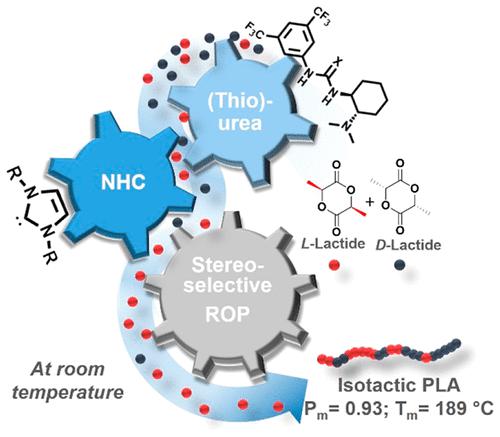
Stereochemical control during polymerization is a key strategy of polymer chemistry to achieve semicrystalline engineered plastics. The stereoselective ring-opening polymerization (ROP) of racemic lactide (rac-LA), which can lead to highly isotactic polylactide (PLA), is one of the emblematic examples in this area. Surprisingly, stereoselective ROP of rac-LA employing chiral organocatalysts has been under-leveraged. Here we show that a commercially available chiral thiourea (TU1), or its urea homologue (U1), can be used in conjunction with an appropriately selected N-heterocyclic carbene (NHC) to trigger the stereoselective ROP of rac-LA at room temperature in toluene. Both a high organic catalysis activity (>90% monomer conversion in 5–9 h) and a high stereoselectivity (probability of formation of meso dyads, Pm, in the range 0.82–0.93) can be achieved by thus pairing a NHC and a chiral amino(thio)urea. The less sterically hindered and the more basic NHC, that is, a NHC bearing tert-butyl substituents (NHCtBu), provides the highest stereoselectivity when employed in conjunction with the chiral TU1 or U1. This asymmetric organic catalysis strategy, as applied here in polymerization chemistry, further expands the field of possibilities to achieve bioplastics with adapted thermomechanical properties.
中文翻译:

联合使用手性(硫代)脲和 N-杂环卡宾实现外消旋丙交酯有机催化开环聚合的高立体选择性和高活性
聚合过程中的立体化学控制是聚合物化学实现半结晶工程塑料的关键策略。外消旋丙交酯 ( rac -LA)的立体选择性开环聚合 (ROP)可以生成高度全同立构的聚丙交酯 (PLA),是该领域的标志性例子之一。令人惊讶的是,使用手性有机催化剂的rac -LA的立体选择性 ROP一直未充分利用。Here we show that a commercially available chiral thiourea (TU1), or its urea homologue (U1), can be used in conjunction with an appropriately selected N -heterocyclic carbene (NHC) to trigger the stereoselective ROP of rac-LA 在室温下在甲苯中。通过将NHC和手性氨基(硫代)脲。当与手性 TU1 或 U1 结合使用时,空间位阻较小且碱性更强的 NHC,即带有叔丁基取代基 (NHC tBu ) 的 NHC 可提供最高的立体选择性。这种在聚合化学中应用的不对称有机催化策略进一步扩大了获得具有适应热机械性能的生物塑料的可能性领域。
更新日期:2022-09-06
中文翻译:

联合使用手性(硫代)脲和 N-杂环卡宾实现外消旋丙交酯有机催化开环聚合的高立体选择性和高活性
聚合过程中的立体化学控制是聚合物化学实现半结晶工程塑料的关键策略。外消旋丙交酯 ( rac -LA)的立体选择性开环聚合 (ROP)可以生成高度全同立构的聚丙交酯 (PLA),是该领域的标志性例子之一。令人惊讶的是,使用手性有机催化剂的rac -LA的立体选择性 ROP一直未充分利用。Here we show that a commercially available chiral thiourea (TU1), or its urea homologue (U1), can be used in conjunction with an appropriately selected N -heterocyclic carbene (NHC) to trigger the stereoselective ROP of rac-LA 在室温下在甲苯中。通过将NHC和手性氨基(硫代)脲。当与手性 TU1 或 U1 结合使用时,空间位阻较小且碱性更强的 NHC,即带有叔丁基取代基 (NHC tBu ) 的 NHC 可提供最高的立体选择性。这种在聚合化学中应用的不对称有机催化策略进一步扩大了获得具有适应热机械性能的生物塑料的可能性领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号