Chemical Geology ( IF 3.6 ) Pub Date : 2022-09-03 , DOI: 10.1016/j.chemgeo.2022.121089 Samuel B. Strohm , Sebastian E. Inckemann , Kun Gao , Michael Schweikert , Marie-Louise Lemloh , Wolfgang W. Schmahl , Guntram Jordan
|
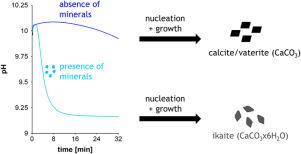
The formation of ikaite (CaCO3x6H2O) was studied in the presence and absence of quartz and mica surfaces using desupersaturation curves from cryo-mixed-batch-reactor experiments. Upon nucleation and growth within the reactor, the solution approached solubility of the precipitating carbonate phase. For ikaite, a solubility constant of log Ksp ikaite = −7.3 ± 0.1 was found (T = 0 °C). At supersaturations Ωikaite < 15, the nucleation of ikaite was significantly promoted by the presence of quartz or mica. This promotion prevented a competing nucleation of anhydrous calcium carbonates. In the presence of quartz or mica, therefore, ikaite forms over a much broader supersaturation range than in the absence. Similarly strong promotors of ikaite nucleation rather than anhydrous carbonate nucleation were previously attributed to calcite-inhibiting substances only.
At supersaturations Ωikaite ≥ 8, application of classical nucleation theory on induction periods of ikaite formation yielded an effective interfacial energy of 15 ± 3 mJ/m2. Compared to data of anhydrous CaCO3 phases, this interfacial energy is low and expresses the highly hydrated character of ikaite. At supersaturations Ωikaite ≥ 18, a transient amorphous phase appeared besides ikaite.
Our results show that a comprehensive understanding of ikaite formation in natural settings requires consideration not only of supersaturation and presence of calcite-inhibitors but also of the presence or absence of mineral surfaces capable of promoting heterogeneous nucleation of ikaite.
中文翻译:

关于 ikaite (CaCO3x6H2O) 的成核 – 存在和不存在矿物表面的比较研究
使用来自低温混合间歇反应器实验的去过饱和曲线研究了在存在和不存在石英和云母表面的情况下ikaite (CaCO 3 x6H 2 O) 的形成。在反应器内成核和生长后,溶液接近沉淀碳酸盐相的溶解度。对于 ikaite,溶解度常数为 log K sp ikaite = -7.3 ± 0.1 ( T = 0 °C)。在过饱和Ω ikaite < 15,石英或云母的存在显着促进了ikaite的成核。这种促进防止了无水碳酸钙的竞争成核。因此,在存在石英或云母的情况下,ikaite 在比不存在时更宽的过饱和范围内形成。类似地,ikaite 成核而不是无水碳酸盐成核的强促进剂以前仅归因于方解石抑制物质。
在过饱和Ω ikaite ≥ 8 时,将经典成核理论应用于 ikaite 形成的诱导期产生了 15 ± 3 mJ/m 2的有效界面能。与无水CaCO 3相的数据相比,该界面能较低,表现出ikaite的高度水合特性。在过饱和Ω ikaite ≥ 18 时,除了 ikaite 之外还出现了一个瞬态非晶相。
我们的研究结果表明,全面了解自然环境中 ikaite 的形成不仅需要考虑过饱和度和方解石抑制剂的存在,还需要考虑是否存在能够促进 ikaite 异质成核的矿物表面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号