当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium Solubility of 18β-Glycyrrhetinic Acid in 12 Pure Solvents: Determination, Correlation, and Hansen Solubility Parameter
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-31 , DOI: 10.1021/acs.jced.2c00399 Yanmin Shen 1 , Ruoyu Li 1 , Peixia Zhao 1 , Wenju Liu 1 , Xiaolong Yang 1 , Zheng Zhang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-31 , DOI: 10.1021/acs.jced.2c00399 Yanmin Shen 1 , Ruoyu Li 1 , Peixia Zhao 1 , Wenju Liu 1 , Xiaolong Yang 1 , Zheng Zhang 1
Affiliation
|
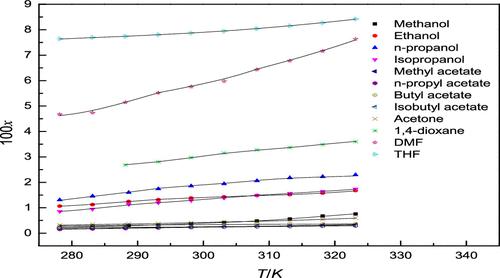
In this study, the solubility data of 18β-glycyrrhetinic acid (GA) in 12 neat solvents were determined by the static equilibrium method at a temperature range of 278.15–323.15 K under a pressure of 0.1 MPa. These selected solvents were methanol, ethanol, n-propanol, isopropanol, methyl acetate, propyl acetate, butyl acetate, isobutyl acetate, acetone, 1,4-dioxane, N,N-dimethylformamide (DMF), and tetrahydrofuran (THF). The experimental results showed that the solubility of GA was positively correlated with temperature and was correlated using thermodynamic models. Activity coefficient models such as the NRTL model, Wilson model, Margules model, and van Laar model were applied to fit the experimental solubility of GA. A good relevance can be obtained for data fitting using these models, and the best fitting effect was achieved with the Wilson equation from ARD and R2. The Hansen solubility parameter was applied to explain the GA solubility phenomenon in pure solvents. These study results contribute to crystallization operation of GA for purification and separation.
中文翻译:

18β-甘草次酸在 12 种纯溶剂中的平衡溶解度:测定、相关性和汉森溶解度参数
在本研究中,18β-甘草次酸(GA)在 12 种纯溶剂中的溶解度数据采用静态平衡法在 278.15-323.15 K 温度范围内,0.1 MPa 压力下测定。这些选定的溶剂是甲醇、乙醇、正丙醇、异丙醇、乙酸甲酯、乙酸丙酯、乙酸丁酯、乙酸异丁酯、丙酮、1,4-二恶烷、N、N-二甲基甲酰胺 (DMF) 和四氢呋喃 (THF)。实验结果表明,GA的溶解度与温度呈正相关,并使用热力学模型进行相关。应用NRTL模型、Wilson模型、Margules模型和van Laar模型等活度系数模型来拟合GA的实验溶解度。使用这些模型可以获得良好的数据拟合相关性,并且使用ARD和R 2的Wilson方程实现了最佳拟合效果。Hansen 溶解度参数用于解释纯溶剂中的 GA 溶解现象。这些研究结果有助于GA的结晶操作用于纯化和分离。
更新日期:2022-08-31
中文翻译:

18β-甘草次酸在 12 种纯溶剂中的平衡溶解度:测定、相关性和汉森溶解度参数
在本研究中,18β-甘草次酸(GA)在 12 种纯溶剂中的溶解度数据采用静态平衡法在 278.15-323.15 K 温度范围内,0.1 MPa 压力下测定。这些选定的溶剂是甲醇、乙醇、正丙醇、异丙醇、乙酸甲酯、乙酸丙酯、乙酸丁酯、乙酸异丁酯、丙酮、1,4-二恶烷、N、N-二甲基甲酰胺 (DMF) 和四氢呋喃 (THF)。实验结果表明,GA的溶解度与温度呈正相关,并使用热力学模型进行相关。应用NRTL模型、Wilson模型、Margules模型和van Laar模型等活度系数模型来拟合GA的实验溶解度。使用这些模型可以获得良好的数据拟合相关性,并且使用ARD和R 2的Wilson方程实现了最佳拟合效果。Hansen 溶解度参数用于解释纯溶剂中的 GA 溶解现象。这些研究结果有助于GA的结晶操作用于纯化和分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号