当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibrium for Ternary Systems of n-Octanol, Ethylene Glycol, and Different Extractants at 298.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-31 , DOI: 10.1021/acs.jced.2c00418 Yingmin Yu 1 , Fangyu Zhang 1 , Xiaocheng Zhang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-31 , DOI: 10.1021/acs.jced.2c00418 Yingmin Yu 1 , Fangyu Zhang 1 , Xiaocheng Zhang 1
Affiliation
|
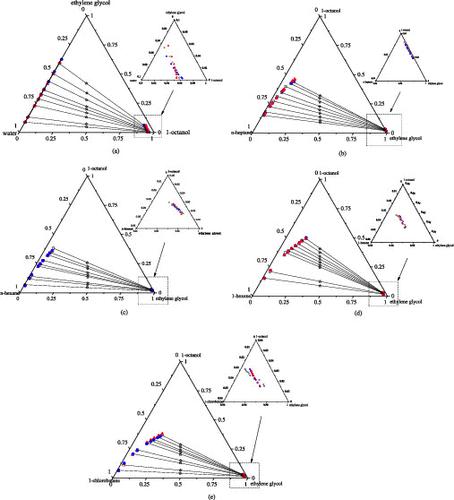
The liquid–liquid equilibrium (LLE) data for the ternary systems of {n-octanol + ethylene glycol + solvents (water/n-heptane/n-hexane/1-hexene/1-chlorobutane)} at 298.2 K under 101.3 kPa were determined. The solute distribution constant and selectivity were used to analyze the solvent’s extraction ability. Meanwhile, the NRTL and UNIQUAC models were applied to correlate the experimental LLE data, and the binary interaction parameters were obtained. The root mean square deviation (RMSD) between the experimental data measured and the calculated by the NRTL and UNIQUAC models was less than 3%. The binary interaction parameters have been confirmed by GUI-MATLAB.
中文翻译:

正辛醇、乙二醇和不同萃取剂三元体系在 298.2 K 下的液-液平衡
{正辛醇 + 乙二醇 + 溶剂 (水/正庚烷/正己烷/1-己烯/1-氯丁烷)}三元体系在 298.2 K 和 101.3 kPa 下的液液平衡 (LLE) 数据为决定。溶质分布常数和选择性用于分析溶剂的萃取能力。同时,应用NRTL和UNIQUAC模型对实验LLE数据进行关联,得到二元相互作用参数。测量的实验数据与 NRTL 和 UNIQUAC 模型计算的均方根偏差 (RMSD) 小于 3%。二进制交互参数已由 GUI-MATLAB 确认。
更新日期:2022-08-31
中文翻译:

正辛醇、乙二醇和不同萃取剂三元体系在 298.2 K 下的液-液平衡
{正辛醇 + 乙二醇 + 溶剂 (水/正庚烷/正己烷/1-己烯/1-氯丁烷)}三元体系在 298.2 K 和 101.3 kPa 下的液液平衡 (LLE) 数据为决定。溶质分布常数和选择性用于分析溶剂的萃取能力。同时,应用NRTL和UNIQUAC模型对实验LLE数据进行关联,得到二元相互作用参数。测量的实验数据与 NRTL 和 UNIQUAC 模型计算的均方根偏差 (RMSD) 小于 3%。二进制交互参数已由 GUI-MATLAB 确认。











































 京公网安备 11010802027423号
京公网安备 11010802027423号