Chemical Engineering and Processing: Process Intensification ( IF 3.8 ) Pub Date : 2022-08-28 , DOI: 10.1016/j.cep.2022.109123 Hamdan Y. Hamdan , Ghassan H. Abdullah
|
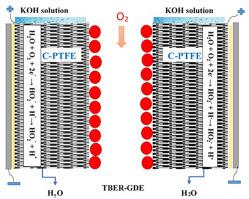
A specific and environmentally benign electrochemical reactor that combines the property of gas diffusion electrode (GDE) with trickling flow property in trickle bed electrochemical reactor (TBER) is designed for intensive hydrogen peroxide (H2O2) production. Compared with other reactors a significant feature of this reactor is the capability to uniformly distribute the oxygen gas all over the cathode bed avoiding oxygen gas transport limitations, electrode limited area, and continuous electrolyte leakage. This reactor consists of two plates as anodes and two porous layers composed of carbon black and polytetrafluoroethylene (C-PTFE) mixture that are pasted on two stainless steel meshes(SSM) to form cathode beds (C-PTFE-SSM). The cathodes are placed on both cathode frame sides which were designed to comprise two separated cathodes. The oxygen gas can flow easily through the space between cathodes and diffuses to the solid-liquid interface to be electrochemically reduced to form H2O2. H2O2 was in situ electrosynthesized in an alkaline solution by the oxygen electrochemical reduction on the cathode surface. All operating conditions that affect the process were systematically investigated. The highest concentration of H2O2 of 46.56 mM was achieved in 40 min and 1.0 V at room temperature, whereas at 0 °C the generated peroxide was 57.86 mM. This significant performance is attributed to the uniform oxygen distribution on the electrode bed making an easy access for gas transport to the electrolyte-cathode interface. The low temperature increases oxygen solubility which increases the production rate as well. Power consumption was also estimated and found to be 4 kWh/kg which is economically beneficial. At different applied cell voltages and best-operating conditions, a kinetic study was conducted for H2O2 electrosynthesized.
中文翻译:

一种用于高效生产过氧化氢的新型滴流床电化学反应器设计
一种结合气体扩散电极 (GDE) 特性和滴流床电化学反应器 (TBER) 中的滴流特性的特殊且环保的电化学反应器,旨在用于高强度过氧化氢 (H 2 O 2 )) 生产。与其他反应器相比,该反应器的一个显着特点是能够将氧气均匀分布在整个阴极床层,避免氧气传输限制、电极受限区域和连续电解质泄漏。该反应器由两块板作为阳极和两个由炭黑和聚四氟乙烯 (C-PTFE) 混合物组成的多孔层组成,它们粘贴在两个不锈钢网 (SSM) 上以形成阴极床 (C-PTFE-SSM)。阴极放置在设计成包括两个分开的阴极的阴极框架两侧。氧气很容易流过阴极之间的空间,扩散到固液界面,被电化学还原形成H 2 O 2。H 2 O2在碱性溶液中通过阴极表面上的氧电化学还原原位电合成。系统地研究了影响该过程的所有操作条件。H 2 O 2最高浓度在室温下 40 分钟和 1.0 V 达到 46.56 mM,而在 0 °C 下生成的过氧化物为 57.86 mM。这种显着的性能归因于电极床上的均匀氧气分布,使气体易于进入电解质-阴极界面。低温增加了氧的溶解度,这也增加了生产率。还估计了功耗并发现为 4 kWh/kg,这在经济上是有利的。在不同的施加电池电压和最佳操作条件下,对电合成的 H 2 O 2进行了动力学研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号