Journal of Advanced Research ( IF 11.4 ) Pub Date : 2022-08-27 , DOI: 10.1016/j.jare.2022.08.006 Zongli Zhang 1 , Yue Yuan 2 , Lin Hu 1 , Jian Tang 1 , Zhongji Meng 3 , Longjun Dai 4 , Yujiu Gao 1 , Shinan Ma 5 , Xiaoli Wang 5 , Yahong Yuan 5 , Qiufang Zhang 5 , Weibin Cai 6 , Xuzhi Ruan 1 , Xingrong Guo 1
|
Introduction
High calorie intake is known to induce nonalcoholic fatty liver disease (NAFLD) by promoting chronic inflammation. However, the mechanisms are poorly understood.
Objectives
This study examined the roles of ANGPTL8 in the regulation of NAFLD-associated liver fibrosis progression induced by high fat diet (HFD)-mediated inflammation.
Methods
The ANGPTL8 concentration was measured in serum samples from liver cancer and liver cirrhosis patients. ANGPTL8 knockout(KO) mice were used to induce disease models (HFD, HFHC and CCL4) followed by pathological staining, western blot and immunohistochemistry. Hydrodynamic injection of an adeno-associated virus 8 (AAV8) was used to establish a model for restoring ANGPTL8 expression specifically in ANGPTL8 KO mice livers. RNA-sequencing, protein array, Co-IP, etc. were used to study ANGPTL8′s mechanisms in regulating liver fibrosis progression, and drug screening was used to identify an effective inhibitor of ANGPTL8 expression.
Results
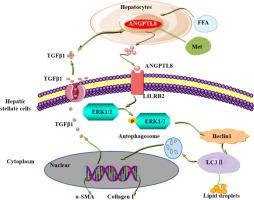
ANGPTL8 level is associated with liver fibrogenesis in both cirrhosis and hepatocellular carcinoma patients. Mouse studies demonstrated that ANGPTL8 deficiency suppresses HFD-stimulated inflammatory activity, hepatic steatosis and liver fibrosis. The AAV-mediated restoration of liver ANGPTL8 expression indicated that liver-derived ANGPTL8 accelerates HFD-induced liver fibrosis. Liver-derived ANGPTL8, as a proinflammatory factor, activates HSCs (hepatic stellate cells) by interacting with the LILRB2 receptor to induce ERK signaling and increase the expression of genes that promote liver fibrosis. The FDA-approved anti-diabetic drug metformin, an ANGPTL8 inhibitor, inhibited HFD-induced liver fibrosis in vivo.
Conclusions
Our data support that ANGPTL8 is a proinflammatory factor that accelerates NAFLD-associated liver fibrosis induced by HFD. The serum ANGPTL8 level may be a potential and specific diagnostic marker for liver fibrosis, and targeting ANGPTL8 holds great promise for developing innovative therapies to treat NAFLD-associated liver fibrosis.
中文翻译:

ANGPTL8 通过 LILRB2/ERK 信号通路加速由 HFD 诱导的炎症活动介导的肝纤维化
介绍
众所周知,高热量摄入会促进慢性炎症,从而诱发非酒精性脂肪性肝病 (NAFLD)。然而,人们对这些机制知之甚少。
目标
本研究检查了 ANGPTL8 在调节由高脂肪饮食 (HFD) 介导的炎症诱导的 NAFLD 相关肝纤维化进程中的作用。
方法
在肝癌和肝硬化患者的血清样本中测量了 ANGPTL8 浓度。ANGPTL8敲除 (KO) 小鼠用于诱导疾病模型(HFD、HFHC 和 CCL4),然后进行病理染色、蛋白质印迹和免疫组织化学。腺相关病毒 8 (AAV8) 的流体动力学注射用于建立模型,用于恢复 ANGPTL8 表达,特别是在ANGPTL8 KO 小鼠肝脏中。采用RNA测序、蛋白芯片、Co-IP等方法研究ANGPTL8调控肝纤维化进程的机制,并通过药物筛选筛选出ANGPTL8表达的有效抑制剂。
结果
ANGPTL8 水平与肝硬化和肝细胞癌患者的肝纤维化相关。小鼠研究表明,ANGPTL8 缺乏可抑制 HFD 刺激的炎症活动、肝脂肪变性和肝纤维化。AAV 介导的肝脏 ANGPTL8 表达恢复表明,肝源性 ANGPTL8 加速了 HFD 诱导的肝纤维化。肝源性 ANGPTL8 作为促炎因子,通过与 LILRB2 受体相互作用来激活 HSC(肝星状细胞),从而诱导 ERK 信号传导并增加促进肝纤维化的基因表达。FDA 批准的抗糖尿病药物二甲双胍是一种 ANGPTL8 抑制剂,可在体内抑制 HFD 诱导的肝纤维化。
结论
我们的数据支持 ANGPTL8 是一种促炎因子,可加速 HFD 诱导的 NAFLD 相关肝纤维化。血清 ANGPTL8 水平可能是肝纤维化的潜在特异性诊断标志物,靶向 ANGPTL8 有望开发出治疗 NAFLD 相关肝纤维化的创新疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号