当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the reliability of atoms in molecules, noncovalent index, and natural bond orbital to identify and quantify noncovalent bonds
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-08-26 , DOI: 10.1002/jcc.26983 Steve Scheiner 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-08-26 , DOI: 10.1002/jcc.26983 Steve Scheiner 1
Affiliation
|
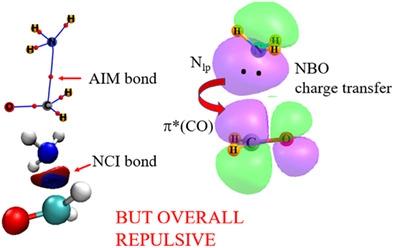
Atoms in molecules, noncovalent index, and natural bond orbital methods are commonly invoked to identify the presence of various noncovalent bonds and to measure their strength. However, there are numerous instances in the literature where these methods provide contradictory or apparently erroneous interpretations of the bonding. The range of reliability of these methods is assessed by calculations of a variety of systems, which include an H-bond, halogen bond, π-tetrel bond, CH··HC interaction, and a pairing of two anions. While the results appear to be meaningful for the equilibrium geometries, and those where the two subunits are progressively pulled apart, these techniques erroneously predict a progressively stronger bonding interaction as the two units are compressed and the interaction becomes clearly repulsive. The methods falsely indicate a bonding interaction in the CH··HC arrangement, and incorrectly mimic the behavior of the energy when two anions approach. These approaches are also unreliable for understanding angular deformations.
中文翻译:

关于分子中原子的可靠性、非共价指数和自然键轨道识别和量化非共价键
分子中的原子、非共价指数和自然键轨道方法通常被用来识别各种非共价键的存在并测量它们的强度。然而,在文献中有许多例子,这些方法对键合提供了矛盾或明显错误的解释。这些方法的可靠性范围是通过对各种系统的计算来评估的,包括氢键、卤素键、π-四方键、CH··HC 相互作用和两个阴离子的配对。虽然结果似乎对平衡几何形状有意义,并且两个亚基逐渐拉开,但这些技术错误地预测随着两个单元被压缩并且相互作用变得明显排斥,这些技术错误地预测了逐渐更强的键合相互作用。这些方法错误地表明了 CH··HC 排列中的键合相互作用,并且错误地模拟了两个阴离子接近时的能量行为。这些方法对于理解角度变形也是不可靠的。
更新日期:2022-08-26
中文翻译:

关于分子中原子的可靠性、非共价指数和自然键轨道识别和量化非共价键
分子中的原子、非共价指数和自然键轨道方法通常被用来识别各种非共价键的存在并测量它们的强度。然而,在文献中有许多例子,这些方法对键合提供了矛盾或明显错误的解释。这些方法的可靠性范围是通过对各种系统的计算来评估的,包括氢键、卤素键、π-四方键、CH··HC 相互作用和两个阴离子的配对。虽然结果似乎对平衡几何形状有意义,并且两个亚基逐渐拉开,但这些技术错误地预测随着两个单元被压缩并且相互作用变得明显排斥,这些技术错误地预测了逐渐更强的键合相互作用。这些方法错误地表明了 CH··HC 排列中的键合相互作用,并且错误地模拟了两个阴离子接近时的能量行为。这些方法对于理解角度变形也是不可靠的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号