当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of Solid–Liquid Phase Equilibria of Na2SO4–(NH4)2SO4–MgSO4–H2O at T = 318.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-26 , DOI: 10.1021/acs.jced.2c00335 Xuanli Xia 1 , Manxin Ding 1 , Yongsheng Ren 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-26 , DOI: 10.1021/acs.jced.2c00335 Xuanli Xia 1 , Manxin Ding 1 , Yongsheng Ren 1, 2
Affiliation
|
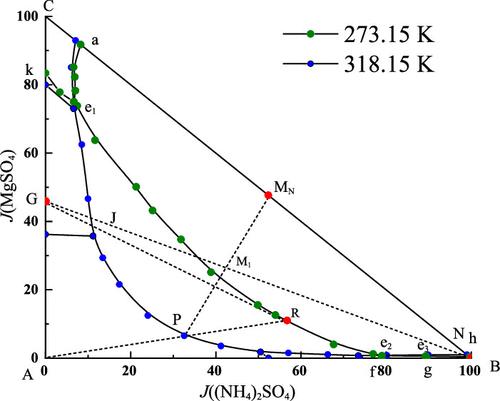
The solubility data of the quaternary system Na+, NH4+, Mg2+∥SO42––H2O and its subsystems Na+, NH4+∥SO42––H2O and Mg2+, NH4+∥SO42––H2O were measured by an isothermal dissolution method at T = 318.15 K. The physical property data (density and viscosity) of the supernatant were also obtained. The experimental results show that both ternary systems contain two invariant points, which are complex systems. The quaternary system (Na+, NH4+, Mg2+∥SO42––H2O) has four invariant points, nine univariate curves, and six crystallization regions. The crystal area of double salt (NH4)2Mg(SO4)2·6H2O is the largest, and it is the easiest to crystallize from the saturated solution. Density and viscosity varies regularly with solution composition in each system. The translation method is used to predict the quaternary system, and the results are consistent with the experiment. Based on the phase diagram of Na+, Mg2+, NH4+∥SO42––H2O, the process of producing a nitrogen–magnesium compound fertilizer was designed, which provided a theoretical basis for guiding the industrial crystallization of salt lakes.
中文翻译:

在 T = 318.15 K 时测定 Na2SO4–(NH4)2SO4–MgSO4–H2O 的固液相平衡
四元体系Na + , NH 4 + , Mg 2+ ∥SO 4 2– –H 2 O及其子系统Na + , NH 4 + ∥SO 4 2– –H 2 O和Mg 2+ , NH的溶解度数据4 + ∥SO 4 2- -H 2 O 采用等温溶解法在T= 318.15 K。还获得了上清液的物理性质数据(密度和粘度)。实验结果表明,这两个三元系统都包含两个不变点,都是复系统。四元体系(Na + , NH 4 + , Mg 2+ ∥SO 4 2– -H 2 O)有四个不变点、九个单变量曲线和六个结晶区。复盐晶体面积(NH 4 ) 2 Mg(SO 4 ) 2 ·6H 2O最大,最容易从饱和溶液中结晶出来。密度和粘度随着每个系统中的溶液成分而有规律地变化。采用平移法对四元系进行预测,结果与实验一致。根据Na +、Mg 2+、NH 4 + ∥SO 4 2– -H 2 O的相图设计了一种氮镁复合肥的生产工艺,为指导工业结晶化生产提供了理论依据。盐湖。
更新日期:2022-08-26
中文翻译:

在 T = 318.15 K 时测定 Na2SO4–(NH4)2SO4–MgSO4–H2O 的固液相平衡
四元体系Na + , NH 4 + , Mg 2+ ∥SO 4 2– –H 2 O及其子系统Na + , NH 4 + ∥SO 4 2– –H 2 O和Mg 2+ , NH的溶解度数据4 + ∥SO 4 2- -H 2 O 采用等温溶解法在T= 318.15 K。还获得了上清液的物理性质数据(密度和粘度)。实验结果表明,这两个三元系统都包含两个不变点,都是复系统。四元体系(Na + , NH 4 + , Mg 2+ ∥SO 4 2– -H 2 O)有四个不变点、九个单变量曲线和六个结晶区。复盐晶体面积(NH 4 ) 2 Mg(SO 4 ) 2 ·6H 2O最大,最容易从饱和溶液中结晶出来。密度和粘度随着每个系统中的溶液成分而有规律地变化。采用平移法对四元系进行预测,结果与实验一致。根据Na +、Mg 2+、NH 4 + ∥SO 4 2– -H 2 O的相图设计了一种氮镁复合肥的生产工艺,为指导工业结晶化生产提供了理论依据。盐湖。











































 京公网安备 11010802027423号
京公网安备 11010802027423号