当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A quasi-intercalation reaction for fast sulfur redox kinetics in solid-state lithium–sulfur batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-08-22 , DOI: 10.1039/d2ee01820a Chuang Li 1 , Qi Zhang 1 , Jinzhi Sheng 1 , Biao Chen 1, 2 , Runhua Gao 1 , Zhihong Piao 1 , Xiongwei Zhong 1 , Zhiyuan Han 1 , Yanfei Zhu 1 , Jiulin Wang 3 , Guangmin Zhou 1 , Hui-Ming Cheng 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-08-22 , DOI: 10.1039/d2ee01820a Chuang Li 1 , Qi Zhang 1 , Jinzhi Sheng 1 , Biao Chen 1, 2 , Runhua Gao 1 , Zhihong Piao 1 , Xiongwei Zhong 1 , Zhiyuan Han 1 , Yanfei Zhu 1 , Jiulin Wang 3 , Guangmin Zhou 1 , Hui-Ming Cheng 4, 5
Affiliation
|
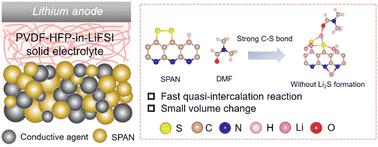
Solid-state lithium–sulfur (Li–S) batteries have been recognized as a competitive candidate for next-generation energy storage systems due to their high energy density and safety. However, the slow redox kinetics between S and Li2S and the large volume change of sulfur during charge/discharge have hindered the development of solid-state Li–S batteries. We report a solid-state Li–S battery using a polymer-in-salt solid-state electrolyte, in which the sulfur is anchored in a polyacrylonitrile (PAN) substrate during cycling, avoiding the formation of Li2S and thus resulting in much faster redox kinetics and a smaller volume change than the conventional solid-state Li–S batteries. The quasi-intercalation reaction in the system is achieved with the assistance of the residual N,N-dimethylformamide (DMF), which helps strengthen the C–S bond. As a result, the solid-state Li-sulfurized PAN (SPAN) batteries have a superb rate capability at room temperature, even higher than those of liquid-state Li–S batteries, due to the faster redox kinetics and smaller volume change without solid–solid S to Li2S conversion which is present in liquid-state Li–SPAN batteries. This is the first report of the redox kinetics of solid-state Li–SPAN batteries being increased by changing the bond-strength of the C–S bond instead of using catalysts. This technique opens up new opportunities for designing high-performance solid-state Li–S batteries.
中文翻译:

固态锂硫电池中快速硫氧化还原动力学的准插层反应
固态锂硫 (Li-S) 电池因其高能量密度和安全性而被公认为下一代储能系统的竞争候选者。然而,S和Li 2 S之间缓慢的氧化还原动力学以及充放电过程中硫的大体积变化阻碍了固态Li-S电池的发展。我们报告了一种使用盐包聚合物固态电解质的固态 Li-S 电池,其中硫在循环过程中被锚定在聚丙烯腈 (PAN) 基底中,避免了 Li 2 S 的形成,从而导致大量比传统的固态锂硫电池更快的氧化还原动力学和更小的体积变化。系统中的准插层反应是在残余N的帮助下实现的, N-二甲基甲酰胺 (DMF),有助于加强 C-S 键。因此,固态锂硫化 PAN (SPAN) 电池在室温下具有出色的倍率性能,甚至高于液态 Li-S 电池,这是由于在没有固态的情况下具有更快的氧化还原动力学和更小的体积变化。 –固态 S 到 Li 2 S 的转化,存在于液态 Li-SPAN 电池中。这是通过改变 C-S 键的键强度而不是使用催化剂来增加固态 Li-SPAN 电池的氧化还原动力学的第一份报告。该技术为设计高性能固态锂硫电池开辟了新机遇。
更新日期:2022-08-22
中文翻译:

固态锂硫电池中快速硫氧化还原动力学的准插层反应
固态锂硫 (Li-S) 电池因其高能量密度和安全性而被公认为下一代储能系统的竞争候选者。然而,S和Li 2 S之间缓慢的氧化还原动力学以及充放电过程中硫的大体积变化阻碍了固态Li-S电池的发展。我们报告了一种使用盐包聚合物固态电解质的固态 Li-S 电池,其中硫在循环过程中被锚定在聚丙烯腈 (PAN) 基底中,避免了 Li 2 S 的形成,从而导致大量比传统的固态锂硫电池更快的氧化还原动力学和更小的体积变化。系统中的准插层反应是在残余N的帮助下实现的, N-二甲基甲酰胺 (DMF),有助于加强 C-S 键。因此,固态锂硫化 PAN (SPAN) 电池在室温下具有出色的倍率性能,甚至高于液态 Li-S 电池,这是由于在没有固态的情况下具有更快的氧化还原动力学和更小的体积变化。 –固态 S 到 Li 2 S 的转化,存在于液态 Li-SPAN 电池中。这是通过改变 C-S 键的键强度而不是使用催化剂来增加固态 Li-SPAN 电池的氧化还原动力学的第一份报告。该技术为设计高性能固态锂硫电池开辟了新机遇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号