Journal of Hepatology ( IF 26.8 ) Pub Date : 2022-08-19 , DOI: 10.1016/j.jhep.2022.08.008 Anna Hadjihambi 1 , Christos Konstantinou 2 , Jan Klohs 3 , Katia Monsorno 4 , Adrien Le Guennec 5 , Chris Donnelly 6 , I Jane Cox 2 , Anjali Kusumbe 7 , Patrick S Hosford 8 , Ugo Soffientini 2 , Salvatore Lecca 9 , Manuel Mameli 10 , Rajiv Jalan 11 , Rosa Chiara Paolicelli 4 , Luc Pellerin 12
|
Background & Aims
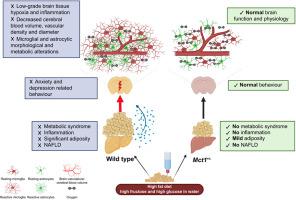
Non-alcoholic fatty liver disease (NAFLD) has been associated with mild cerebral dysfunction and cognitive decline, although the exact pathophysiological mechanism remains ambiguous. Using a diet-induced model of NAFLD and monocarboxylate transporter-1 (Mct1+/−) haploinsufficient mice, which resist high-fat diet-induced hepatic steatosis, we investigated the hypothesis that NAFLD leads to an encephalopathy by altering cognition, behaviour, and cerebral physiology. We also proposed that global MCT1 downregulation offers cerebral protection.
Methods
Behavioural tests were performed in mice following 16 weeks of control diet (normal chow) or high-fat diet with high fructose/glucose in water. Tissue oxygenation, cerebrovascular reactivity, and cerebral blood volume were monitored under anaesthesia by multispectral optoacoustic tomography and optical fluorescence. Cortical mitochondrial oxygen consumption and respiratory capacities were measured using ex vivo high-resolution respirometry. Microglial and astrocytic changes were evaluated by immunofluorescence and 3D reconstructions. Body composition was assessed using EchoMRI, and liver steatosis was confirmed by histology.
Results
NAFLD concomitant with obesity is associated with anxiety- and depression-related behaviour. Low-grade brain tissue hypoxia was observed, likely attributed to the low-grade brain inflammation and decreased cerebral blood volume. It is also accompanied by microglial and astrocytic morphological and metabolic alterations (higher oxygen consumption), suggesting the early stages of an obesogenic diet-induced encephalopathy. Mct1 haploinsufficient mice, despite fat accumulation in adipose tissue, were protected from NAFLD and associated cerebral alterations.
Conclusions
This study provides evidence of compromised brain health in obesity and NAFLD, emphasising the importance of the liver–brain axis. The protective effect of Mct1 haploinsufficiency points to this protein as a novel therapeutic target for preventing and/or treating NAFLD and the associated brain dysfunction.
Impact and implications
This study is focused on unravelling the pathophysiological mechanism by which cerebral dysfunction and cognitive decline occurs during NAFLD and exploring the potential of monocarboxylate transporter-1 (MCT1) as a novel preventive or therapeutic target. Our findings point to NAFLD as a serious health risk and its adverse impact on the brain as a potential global health system and economic burden. These results highlight the utility of Mct1 transgenic mice as a model for NAFLD and associated brain dysfunction and call for systematic screening by physicians for early signs of psychological symptoms, and an awareness by individuals at risk of these potential neurological effects. This study is expected to bring attention to the need for early diagnosis and treatment of NAFLD, while having a direct impact on policies worldwide regarding the health risk associated with NAFLD, and its prevention and treatment.
中文翻译:

部分 MCT1 失效可预防饮食引起的非酒精性脂肪肝和相关的脑功能障碍
背景与目标
非酒精性脂肪性肝病 (NAFLD) 与轻度脑功能障碍和认知能力下降有关,但确切的病理生理机制仍不明确。我们使用饮食诱导的 NAFLD 模型和单羧酸转运蛋白-1 ( Mct1 +/- ) 单倍剂量不足的小鼠来抵抗高脂肪饮食诱导的肝脂肪变性,我们研究了 NAFLD 通过改变认知、行为和行为导致脑病的假设。脑生理学。我们还提出全球 MCT1 下调提供大脑保护。
方法
在控制饮食(正常食物)或含有高果糖/葡萄糖的高脂肪饮食 16 周后,对小鼠进行了行为测试。通过多光谱光声断层扫描和光学荧光在麻醉下监测组织氧合、脑血管反应性和脑血容量。使用体外高分辨率呼吸测量法测量皮质线粒体耗氧量和呼吸能力。通过免疫荧光和 3D 重建评估小胶质细胞和星形胶质细胞的变化。使用 EchoMRI 评估身体成分,并通过组织学证实肝脏脂肪变性。
结果
伴随肥胖的 NAFLD 与焦虑和抑郁相关的行为有关。观察到低度脑组织缺氧,可能归因于低度脑炎症和脑血容量减少。它还伴随着小胶质细胞和星形胶质细胞的形态和代谢改变(更高的耗氧量),表明肥胖饮食诱发脑病的早期阶段。Mct1单倍体不足的小鼠,尽管脂肪组织中有脂肪堆积,但免受 NAFLD 和相关脑改变的影响。
结论
这项研究提供了肥胖和 NAFLD 患者大脑健康受损的证据,强调了肝脑轴的重要性。Mct1单倍剂量不足的保护作用表明该蛋白可作为预防和/或治疗 NAFLD 和相关脑功能障碍的新型治疗靶点。
影响和启示
本研究的重点是阐明 NAFLD 期间发生脑功能障碍和认知能力下降的病理生理机制,并探索单羧酸转运蛋白 1 (MCT1) 作为新型预防或治疗靶点的潜力。我们的研究结果表明,NAFLD 是一种严重的健康风险,它对大脑的不利影响是潜在的全球卫生系统和经济负担。这些结果突出了Mct1的效用转基因小鼠作为 NAFLD 和相关脑功能障碍的模型,并呼吁医生对心理症状的早期迹象进行系统筛查,并呼吁个人意识到这些潜在神经系统影响的风险。这项研究有望引起人们对 NAFLD 早期诊断和治疗需求的关注,同时对全球有关与 NAFLD 相关的健康风险及其预防和治疗的政策产生直接影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号