当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peroxy Intermediate Drives Carbon Bond Activation in the Dioxygenase AsqJ
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-18 , DOI: 10.1021/jacs.2c05650 Dirk Auman 1 , Felix Ecker 2 , Sophie L Mader 1 , Kevin M Dorst 3 , Alois Bräuer 2 , Göran Widmalm 3 , Michael Groll 2 , Ville R I Kaila 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-18 , DOI: 10.1021/jacs.2c05650 Dirk Auman 1 , Felix Ecker 2 , Sophie L Mader 1 , Kevin M Dorst 3 , Alois Bräuer 2 , Göran Widmalm 3 , Michael Groll 2 , Ville R I Kaila 1
Affiliation
|
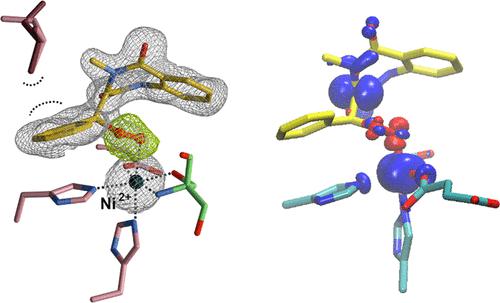
Dioxygenases catalyze stereoselective oxygen atom transfer in metabolic pathways of biological, industrial, and pharmaceutical importance, but their precise chemical principles remain controversial. The α-ketoglutarate (αKG)-dependent dioxygenase AsqJ synthesizes biomedically active quinolone alkaloids via desaturation and subsequent epoxidation of a carbon–carbon bond in the cyclopeptin substrate. Here, we combine high-resolution X-ray crystallography with enzyme engineering, quantum-classical (QM/MM) simulations, and biochemical assays to describe a peroxidic intermediate that bridges the substrate and active site metal ion in AsqJ. Homolytic cleavage of this moiety during substrate epoxidation generates an activated high-valent ferryl (FeIV = O) species that mediates the next catalytic cycle, possibly without the consumption of the metabolically valuable αKG cosubstrate. Our combined findings provide an important understanding of chemical bond activation principles in complex enzymatic reaction networks and molecular mechanisms of dioxygenases.
中文翻译:

过氧中间体驱动双加氧酶 AsqJ 中的碳键活化
双加氧酶在具有生物学、工业和药学重要性的代谢途径中催化立体选择性氧原子转移,但它们的精确化学原理仍存在争议。α-酮戊二酸 (αKG) 依赖性双加氧酶 AsqJ 通过去饱和和随后环肽素底物中碳-碳键的环氧化来合成具有生物医学活性的喹诺酮类生物碱。在这里,我们将高分辨率 X 射线晶体学与酶工程、量子经典 (QM/MM) 模拟和生化测定相结合,描述了一种过氧化物中间体,它在 AsqJ 中桥接底物和活性位点金属离子。在底物环氧化过程中该部分的均裂裂解产生活化的高价ferrl(Fe IV= O) 介导下一个催化循环的物质,可能不消耗具有代谢价值的 αKG 共底物。我们的综合研究结果为复杂酶促反应网络中的化学键活化原理和双加氧酶的分子机制提供了重要的理解。
更新日期:2022-08-18
中文翻译:

过氧中间体驱动双加氧酶 AsqJ 中的碳键活化
双加氧酶在具有生物学、工业和药学重要性的代谢途径中催化立体选择性氧原子转移,但它们的精确化学原理仍存在争议。α-酮戊二酸 (αKG) 依赖性双加氧酶 AsqJ 通过去饱和和随后环肽素底物中碳-碳键的环氧化来合成具有生物医学活性的喹诺酮类生物碱。在这里,我们将高分辨率 X 射线晶体学与酶工程、量子经典 (QM/MM) 模拟和生化测定相结合,描述了一种过氧化物中间体,它在 AsqJ 中桥接底物和活性位点金属离子。在底物环氧化过程中该部分的均裂裂解产生活化的高价ferrl(Fe IV= O) 介导下一个催化循环的物质,可能不消耗具有代谢价值的 αKG 共底物。我们的综合研究结果为复杂酶促反应网络中的化学键活化原理和双加氧酶的分子机制提供了重要的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号