当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Halogen Atom Participation in Guiding the Stereochemical Outcomes of Acetal Substitution Reactions
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-08-18 , DOI: 10.1002/anie.202209401 Krystyna M Demkiw 1 , Wouter A Remmerswaal 2 , Thomas Hansen 2 , Gijsbert A van der Marel 2 , Jeroen D C Codée 2 , K A Woerpel 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-08-18 , DOI: 10.1002/anie.202209401 Krystyna M Demkiw 1 , Wouter A Remmerswaal 2 , Thomas Hansen 2 , Gijsbert A van der Marel 2 , Jeroen D C Codée 2 , K A Woerpel 1
Affiliation
|
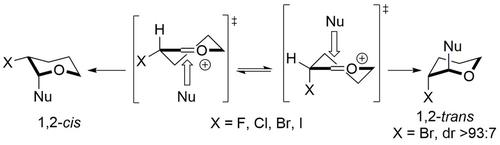
Nucleophilic additions to α-haloacetals proceed via an oxocarbenium ion intermediate where the carbon-halogen bond can either adopt a pseudo-axial or a pseudo-equatorial position. trans-Selectivity is typically observed when a halogen atom is positioned at C-2 of an acetal because hyperconjugative interactions involving the pseudo-axial carbon-halogen bond stabilize the oxocarbenium ion intermediate.
中文翻译:

卤素原子参与指导缩醛取代反应的立体化学结果
α-卤缩醛的亲核加成通过氧碳正离子中间体进行,其中碳-卤素键可以采用伪轴向或伪赤道位置。当卤素原子位于缩醛的 C-2 时,通常会观察到反式选择性,因为涉及假轴向碳-卤素键的超共轭相互作用稳定了氧碳正离子中间体。
更新日期:2022-08-18
中文翻译:

卤素原子参与指导缩醛取代反应的立体化学结果
α-卤缩醛的亲核加成通过氧碳正离子中间体进行,其中碳-卤素键可以采用伪轴向或伪赤道位置。当卤素原子位于缩醛的 C-2 时,通常会观察到反式选择性,因为涉及假轴向碳-卤素键的超共轭相互作用稳定了氧碳正离子中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号