Cell Metabolism ( IF 27.7 ) Pub Date : 2022-08-18 , DOI: 10.1016/j.cmet.2022.07.013 Tamer Coskun 1 , Shweta Urva 1 , William C Roell 1 , Hongchang Qu 1 , Corina Loghin 1 , Julie S Moyers 1 , Libbey S O'Farrell 1 , Daniel A Briere 1 , Kyle W Sloop 1 , Melissa K Thomas 1 , Valentina Pirro 1 , David B Wainscott 1 , Francis S Willard 1 , Matthew Abernathy 1 , LaRonda Morford 1 , Yu Du 1 , Charles Benson 1 , Ruth E Gimeno 1 , Axel Haupt 1 , Zvonko Milicevic 2
|
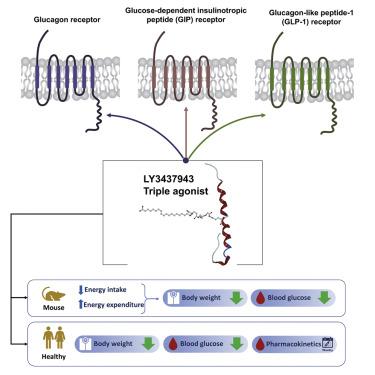
With an increasing prevalence of obesity, there is a need for new therapies to improve body weight management and metabolic health. Multireceptor agonists in development may provide approaches to fulfill this unmet medical need. LY3437943 is a novel triple agonist peptide at the glucagon receptor (GCGR), glucose-dependent insulinotropic polypeptide receptor (GIPR), and glucagon-like peptide-1 receptor (GLP-1R). In vitro, LY3437943 shows balanced GCGR and GLP-1R activity but more GIPR activity. In obese mice, administration of LY3437943 decreased body weight and improved glycemic control. Body weight loss was augmented by the addition of GCGR-mediated increases in energy expenditure to GIPR- and GLP-1R-driven calorie intake reduction. In a phase 1 single ascending dose study, LY3437943 showed a safety and tolerability profile similar to other incretins. Its pharmacokinetic profile supported once-weekly dosing, and a reduction in body weight persisted up to day 43 after a single dose. These findings warrant further clinical assessment of LY3437943.
中文翻译:

LY3437943,一种用于血糖控制和减肥的新型胰高血糖素、GIP 和 GLP-1 受体激动剂:从发现到临床概念验证
随着肥胖症的日益流行,需要新的疗法来改善体重管理和代谢健康。开发中的多受体激动剂可能会提供满足这种未满足的医疗需求的方法。LY3437943 是一种新型三联激动剂肽,作用于胰高血糖素受体 (GCGR)、葡萄糖依赖性促胰岛素多肽受体 (GIPR) 和胰高血糖素样肽-1 受体 (GLP-1R)。体外, LY3437943 表现出平衡的 GCGR 和 GLP-1R 活性,但更多的 GIPR 活性。在肥胖小鼠中,给予 LY3437943 可降低体重并改善血糖控制。通过将 GCGR 介导的能量消耗增加与 GIPR 和 GLP-1R 驱动的卡路里摄入减少相结合,体重减轻得到了增强。在 1 期单次递增剂量研究中,LY3437943 显示出与其他肠促胰岛素相似的安全性和耐受性。其药代动力学特征支持每周给药一次,单次给药后体重减轻持续至第 43 天。这些发现值得对 LY3437943 进行进一步的临床评估。











































 京公网安备 11010802027423号
京公网安备 11010802027423号