当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evolution of bismuth-based metal–organic frameworks for efficient electroreduction of CO2
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-08-18 , DOI: 10.1039/d2ta04485d Lili Li 1 , Xinchen Kang 1, 2 , Meng He 1 , Alena Sheveleva 1, 3 , Kui Hu 1 , Shaojun Xu 4, 5 , Yiqi Zhou 6, 7 , Jin Chen 1 , Sergei Sapchenko 1 , George Whitehead 1 , Iñigo J Vitorica-Yrezabal 1 , Laura Lopez-Odriozola 1 , Louise S Natrajan 1 , Eric J L McInnes 1, 3 , Martin Schröder 1 , Sihai Yang 1 , Floriana Tuna 1, 3
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-08-18 , DOI: 10.1039/d2ta04485d Lili Li 1 , Xinchen Kang 1, 2 , Meng He 1 , Alena Sheveleva 1, 3 , Kui Hu 1 , Shaojun Xu 4, 5 , Yiqi Zhou 6, 7 , Jin Chen 1 , Sergei Sapchenko 1 , George Whitehead 1 , Iñigo J Vitorica-Yrezabal 1 , Laura Lopez-Odriozola 1 , Louise S Natrajan 1 , Eric J L McInnes 1, 3 , Martin Schröder 1 , Sihai Yang 1 , Floriana Tuna 1, 3
Affiliation
|
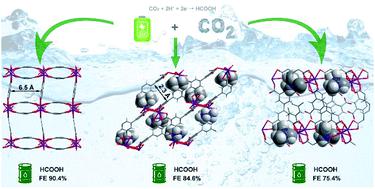
Understanding the structural and chemical changes that reactive metal–organic frameworks (MOFs) undergo is crucial for the development of new efficient catalysts for electrochemical reduction of CO2. Here, we describe three Bi(III) materials, MFM-220, MFM-221 and MFM-222, which are constructed from the same ligand (biphenyl-3,3′,5,5′-tetracarboxylic acid) but which show distinct porosity with solvent-accessible voids of 49.6%, 33.6% and 0%, respectively. We report the first study of the impact of porosity of MOFs on their evolution as electrocatalysts. A Faradaic efficiency of 90.4% at −1.1 V vs. RHE (reversible hydrogen electrode) is observed for formate production over an electrode decorated with MFM-220-p, formed from MFM-220 on application of an external potential in the presence of 0.1 M KHCO3 electrolyte. In situ electron paramagnetic resonance spectroscopy confirms the presence of ·COOH radicals as a reaction intermediate, with an observed stable and consistent Faradaic efficiency and current density for production of formate by electrolysis over 5 h. This study emphasises the significant role of porosity of MOFs as they react and evolve during electroreduction of CO2 to generate value-added chemicals.
中文翻译:

用于有效电还原二氧化碳的铋基金属有机框架的演变
了解活性金属有机框架(MOF)所经历的结构和化学变化对于开发用于电化学还原CO 2的新型高效催化剂至关重要。在这里,我们描述了三种 Bi( III ) 材料,MFM-220、MFM-221 和 MFM-222,它们由相同的配体(联苯-3,3',5,5'-四羧酸)构建,但表现出不同的特性溶剂可及的孔隙率分别为 49.6%、33.6% 和 0%。我们首次研究了 MOF 的孔隙率对其作为电催化剂的演变的影响。在用 MFM-220-p 装饰的电极(由 MFM-220 在存在 0.1 的外部电势的情况下形成)上观察到在 -1.1 V vs. RHE(可逆氢电极)下产生甲酸盐的法拉第效率为 90.4% M KHCO 3电解质。原位电子顺磁共振波谱证实了·COOH自由基作为反应中间体的存在,并观察到通过电解5小时以上稳定一致的法拉第效率和电流密度。这项研究强调了 MOF 的孔隙率在 CO 2电还原过程中发生反应和演化以产生增值化学品时的重要作用。
更新日期:2022-08-18
中文翻译:

用于有效电还原二氧化碳的铋基金属有机框架的演变
了解活性金属有机框架(MOF)所经历的结构和化学变化对于开发用于电化学还原CO 2的新型高效催化剂至关重要。在这里,我们描述了三种 Bi( III ) 材料,MFM-220、MFM-221 和 MFM-222,它们由相同的配体(联苯-3,3',5,5'-四羧酸)构建,但表现出不同的特性溶剂可及的孔隙率分别为 49.6%、33.6% 和 0%。我们首次研究了 MOF 的孔隙率对其作为电催化剂的演变的影响。在用 MFM-220-p 装饰的电极(由 MFM-220 在存在 0.1 的外部电势的情况下形成)上观察到在 -1.1 V vs. RHE(可逆氢电极)下产生甲酸盐的法拉第效率为 90.4% M KHCO 3电解质。原位电子顺磁共振波谱证实了·COOH自由基作为反应中间体的存在,并观察到通过电解5小时以上稳定一致的法拉第效率和电流密度。这项研究强调了 MOF 的孔隙率在 CO 2电还原过程中发生反应和演化以产生增值化学品时的重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号