当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurements of the Heat Capacity of Erbium Titanate Er2Ti2O7
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-18 , DOI: 10.1021/acs.jced.2c00050 Mira R. Bissengaliyeva 1 , Michael A. Bespyatov 2 , Daniil B. Gogol 1 , Daniyar T. Sadyrbekov 1, 3 , Shynar T. Taimassova 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-18 , DOI: 10.1021/acs.jced.2c00050 Mira R. Bissengaliyeva 1 , Michael A. Bespyatov 2 , Daniil B. Gogol 1 , Daniyar T. Sadyrbekov 1, 3 , Shynar T. Taimassova 1
Affiliation
|
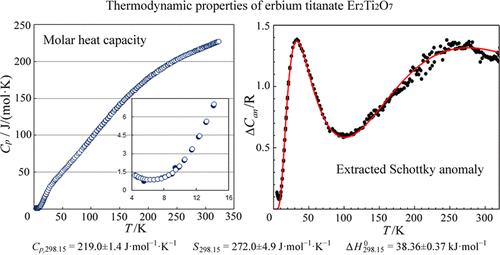
The temperature dependence of the erbium titanate Er2Ti2O7 heat capacity has been studied over the range from 4.5 to 324 K by adiabatic calorimetry. A component related to the presence of the Schottky anomaly has been isolated from the total heat capacity of the compound. Based on the experimental data, the thermodynamic functions of Er2Ti2O7 have been determined over the range from 5 to 320 K. The standard thermodynamic functions of the compound at 298.15 K are as follows: Cp,298.15 = 219.0 ± 1.4 J·mol–1·K–1, S298.15 = 272.0 ± 4.9 J·mol–1·K–1, and ΔH0298.15 = 38.36 ± 0.37 kJ·mol–1.
中文翻译:

钛酸铒 Er2Ti2O7 热容量的测量
钛酸铒 Er 2 Ti 2 O 7热容量的温度依赖性已通过绝热量热法在 4.5 至 324 K 范围内进行了研究。与肖特基异常存在相关的成分已从化合物的总热容量中分离出来。根据实验数据,确定了 Er 2 Ti 2 O 7的热力学函数在 5 至 320 K 范围内。该化合物在 298.15 K 的标准热力学函数如下:C p,298.15 = 219.0 ± 1.4 J·mol –1 ·K –1 , S 298.15 = 272.0 ± 4.9 J·mol –1·K –1,ΔH 0 298.15 = 38.36 ± 0.37 kJ·mol –1。
更新日期:2022-08-18
中文翻译:

钛酸铒 Er2Ti2O7 热容量的测量
钛酸铒 Er 2 Ti 2 O 7热容量的温度依赖性已通过绝热量热法在 4.5 至 324 K 范围内进行了研究。与肖特基异常存在相关的成分已从化合物的总热容量中分离出来。根据实验数据,确定了 Er 2 Ti 2 O 7的热力学函数在 5 至 320 K 范围内。该化合物在 298.15 K 的标准热力学函数如下:C p,298.15 = 219.0 ± 1.4 J·mol –1 ·K –1 , S 298.15 = 272.0 ± 4.9 J·mol –1·K –1,ΔH 0 298.15 = 38.36 ± 0.37 kJ·mol –1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号