当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Thermodynamic Properties of 5-Bromo-2-pyridinecarboxylic Acid in 10 Monosolvents and 3 Binary Solvents at T = (278.15–323.15) K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-17 , DOI: 10.1021/acs.jced.2c00318 Fu Zhu 1 , Peng Zhou 1 , Wenge Yang 1 , Rensong Wang 1 , Chen Chen 1 , Yonghong Hu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-17 , DOI: 10.1021/acs.jced.2c00318 Fu Zhu 1 , Peng Zhou 1 , Wenge Yang 1 , Rensong Wang 1 , Chen Chen 1 , Yonghong Hu 1
Affiliation
|
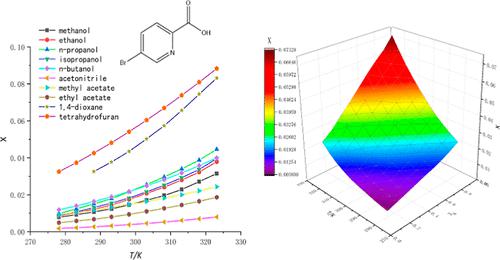
The static equilibrium method combined with high-performance liquid chromatography was used to determine the solubility of 5-bromo-2-pyridinecarboxylic acid at T = 278.15–323.15 K in 10 monosolvents (methanol, ethyl alcohol, acetonitrile, methyl acetate, ethyl acetate, n-propanol, isopropanol, n-butanol, tetrahydrofuran, and 1,4-dioxane) and 3 kinds of binary solvents (ethyl acetate + tetrahydrofuran, ethyl acetate + methanol, and ethyl acetate + 1,4-dioxane). The results show that 5-bromo-2-pyridinecarboxylic acid is positively related to temperature, and the solubility of 5-bromo-2-pyridinecarboxylic acid in tetrahydrofuran was the highest. In monosolvents, the modified Apelblat model and λh model were used to fit the solubility data of 5-bromo-2-pyridinecarboxylic acid. The solubility data of binary solvents were obtained by combining the nearly ideal binary solvent/Redlich–Kister model and Jouyban–Acree model, which showed that the experimental data were in good agreement with the fitting data. Furthermore, we chose the Kamlet, Abboud, and Taft linear solvation energy relationship model to analyze the relationship between the solute–solvent intermolecular interaction and the solubility of 5-bromo-2-pyridinecarboxylic acid. The results show that the increase of solvent–solvent interaction is beneficial to the dissolution of 5-bromo-2-pyridinecarboxylic acid.
中文翻译:

5-溴-2-吡啶甲酸在 10 种单溶剂和 3 种二元溶剂中 T = (278.15–323.15) K 时的溶解度测定和热力学性质
采用静态平衡法结合高效液相色谱法测定 5-溴-2-吡啶甲酸在T = 278.15–323.15 K 时在 10 种单溶剂(甲醇、乙醇、乙腈、乙酸甲酯、乙酸乙酯、正丙醇、异丙醇、正丁醇、四氢呋喃、1,4-二恶烷)和3种二元溶剂(乙酸乙酯+四氢呋喃、乙酸乙酯+甲醇、乙酸乙酯+1,4-二恶烷)。结果表明,5-溴-2-吡啶甲酸与温度呈正相关,5-溴-2-吡啶甲酸在四氢呋喃中的溶解度最高。在单溶剂中,使用改进的 Apelblat 模型和 λh 模型来拟合 5-bromo-2-pyridinecarboxy 酸的溶解度数据。结合近乎理想的二元溶剂/Redlich-Kister模型和Jouyban-Acree模型得到二元溶剂的溶解度数据,表明实验数据与拟合数据吻合较好。此外,我们选择了 Kamlet, Abboud, 和Taft线性溶剂化能量关系模型,分析溶质-溶剂分子间相互作用与5-溴-2-吡啶羧酸溶解度之间的关系。结果表明,溶剂-溶剂相互作用的增加有利于5-溴-2-吡啶甲酸的溶解。
更新日期:2022-08-17
中文翻译:

5-溴-2-吡啶甲酸在 10 种单溶剂和 3 种二元溶剂中 T = (278.15–323.15) K 时的溶解度测定和热力学性质
采用静态平衡法结合高效液相色谱法测定 5-溴-2-吡啶甲酸在T = 278.15–323.15 K 时在 10 种单溶剂(甲醇、乙醇、乙腈、乙酸甲酯、乙酸乙酯、正丙醇、异丙醇、正丁醇、四氢呋喃、1,4-二恶烷)和3种二元溶剂(乙酸乙酯+四氢呋喃、乙酸乙酯+甲醇、乙酸乙酯+1,4-二恶烷)。结果表明,5-溴-2-吡啶甲酸与温度呈正相关,5-溴-2-吡啶甲酸在四氢呋喃中的溶解度最高。在单溶剂中,使用改进的 Apelblat 模型和 λh 模型来拟合 5-bromo-2-pyridinecarboxy 酸的溶解度数据。结合近乎理想的二元溶剂/Redlich-Kister模型和Jouyban-Acree模型得到二元溶剂的溶解度数据,表明实验数据与拟合数据吻合较好。此外,我们选择了 Kamlet, Abboud, 和Taft线性溶剂化能量关系模型,分析溶质-溶剂分子间相互作用与5-溴-2-吡啶羧酸溶解度之间的关系。结果表明,溶剂-溶剂相互作用的增加有利于5-溴-2-吡啶甲酸的溶解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号