Nano Energy ( IF 17.6 ) Pub Date : 2022-08-15 , DOI: 10.1016/j.nanoen.2022.107705 Jiaying Yu , Yongjie Qin , Xiaodeng Wang , Hongju Zheng , Keru Gao , Hengpan Yang , Laiyong Xie , Qi Hu , Chuanxin He
|
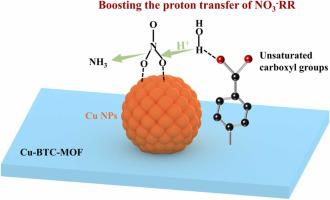
Electrochemical nitrate (NO3-) reduction reaction (NO3-RR) offers an ideal route to harvest ammonia (NH3) under ambient conditions. Despite recent advances in Cu-based NO3-RR electrocatalysts, their synthesis heavily relies on the regulation of adsorption strength towards nitrogen-containing intermediates, and other important factors are ignored (i.e., the proton transfer rate). Here, we select Cu nanoparticles (NPs) as model catalysts to investigate whether and how the proton transfer rate impacts the NO3-RR kinetics. The results indicate that the proton transfer is involved in the rate-determining step (RDS) of NO3-RR, and the weak water dissociation ability of Cu leads to slow proton transfer rate and consequently sluggish NO3-RR kinetics. To this end, we enhance the water dissociation ability of Cu NPs by incorporating uncoordinated carboxylate ligands to enable rapid proton transfer, which in turn boosts the hydrogenation of key intermediates for reducing the overall energy barrier of NO3-RR. As a result, Cu NPs with the ligands display a maximum NH3 yield rate of 496.4 mmol h−1 gcat−1, outperforming counterpart without ligands. This work not only deepens our knowledge on the NO3-RR mechanism, but also offers new guidelines for the smart design of efficient electrocatalysts.
中文翻译:

通过有机配体调谐质子转移促进电化学硝酸盐-氨转化
电化学硝酸盐 (NO 3 - ) 还原反应 (NO 3 - RR) 提供了在环境条件下收获氨 (NH 3 )的理想途径。尽管最近在 Cu 基 NO 3 - RR 电催化剂方面取得了进展,但它们的合成很大程度上依赖于对含氮中间体的吸附强度的调节,而忽略了其他重要因素(即质子转移率)。在这里,我们选择 Cu 纳米粒子 (NPs) 作为模型催化剂来研究质子转移率是否以及如何影响 NO 3 - RR 动力学。结果表明,质子转移参与了 NO 3 -的速率决定步骤(RDS)。RR 和 Cu 的弱水解离能力导致质子转移速率缓慢,从而导致 NO 3 - RR 动力学迟缓。为此,我们通过引入未配位的羧酸盐配体来增强 Cu NPs 的水解离能力,以实现质子的快速转移,进而促进关键中间体的氢化,从而降低 NO 3 - RR 的整体能垒。结果,具有配体的Cu NPs显示出496.4 mmol h -1 g cat -1的最大NH 3产率,优于没有配体的对应物。这项工作不仅加深了我们对 NO 3的认识——RR 机制,但也为高效电催化剂的智能设计提供了新的指导方针。



























 京公网安备 11010802027423号
京公网安备 11010802027423号