当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
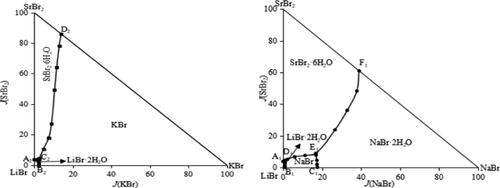
Stable Phase Equilibria in the Quaternary Systems LiBr–NaBr–SrBr2–H2O and LiBr–KBr–SrBr2–H2O at 288.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-15 , DOI: 10.1021/acs.jced.1c00974 Xing-Xing Zeng 1 , Shi-Hua Sang 1 , Xiao-Yun Qi 1 , Xiao-Feng Guo 1 , Rui-Zhi Cui 2, 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-15 , DOI: 10.1021/acs.jced.1c00974 Xing-Xing Zeng 1 , Shi-Hua Sang 1 , Xiao-Yun Qi 1 , Xiao-Feng Guo 1 , Rui-Zhi Cui 2, 3
Affiliation
|
Aiming at the composition of underground brines in the Sichuan Basin of China, stable phase equilibria in quaternary systems LiBr–NaBr–SrBr2–H2O and LiBr–KBr–SrBr2–H2O at 288.15 K are studied using the isothermal dissolution equilibrium method. The solubilities of salts in the quaternary systems listed above are measured, and the corresponding equilibrium solid phases are identified using X-ray powder diffraction. As a result, the corresponding phase diagrams of the two quaternary systems are plotted, respectively. In the two stable phase diagrams, there is no solid solution or complex salt at 288.15 K. For the quaternary system LiBr–NaBr–SrBr2–H2O, there are two invariant points, five univariate curves, and four solid crystallization regions, which are LiBr·2H2O, NaBr, NaBr·2H2O, and SrBr2·6H2O, respectively, of which LiBr·2H2O has the smallest crystallization field, indicating that its solubility is the largest and it is the hardest to be precipitated from the saturated solution. There are one invariant point, three univariate curves, and three solid crystallization fields, corresponding to LiBr·2H2O, KBr, and SrBr2·6H2O in the quaternary system LiBr–KBr–SrBr2–H2O, respectively. The experimental results show that the solubilities of salts KBr, NaBr, and SrBr2 in the saturated solution decrease with the increasing concentration of LiBr, which indicates that LiBr has a strong salting out effect on KBr, NaBr, and SrBr2 in the solution.
更新日期:2022-08-15



























 京公网安备 11010802027423号
京公网安备 11010802027423号