当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A cascade indazolone-directed Ir(III)- and Rh(III)-catalyzed C(sp2)–H functionalization/[4 + 2] annulation of 1-arylindazolones with sulfoxonium ylides to access chemically divergent 8H-indazolo [1,2-a]cinnolines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-08-15 , DOI: 10.1039/d2qo00871h Yi-Chuan Zheng 1 , Bing Shu 2 , Yao-Fu Zeng 3 , Shao-Yong Chen 1 , Jia-Lin Song 1 , Yan-Zhi Liu 1 , Lin Xiao 1 , Xu-Ge Liu 4 , Xuanxuan Zhang 1 , Shang-Shi Zhang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-08-15 , DOI: 10.1039/d2qo00871h Yi-Chuan Zheng 1 , Bing Shu 2 , Yao-Fu Zeng 3 , Shao-Yong Chen 1 , Jia-Lin Song 1 , Yan-Zhi Liu 1 , Lin Xiao 1 , Xu-Ge Liu 4 , Xuanxuan Zhang 1 , Shang-Shi Zhang 1
Affiliation
|
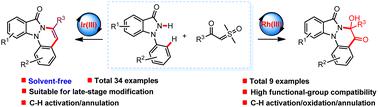
An indazolone-directed chemoselective synthesis of 8H-indazolo [1,2-a]cinnolines has been realized via a cascade Cp*Ir(III)- and Cp*Rh(III)-catalyzed C–H activation/cyclization reaction of 1-arylindazolones with sulfoxonium ylides. The strategy showcased broad substrate scope, high functional group compatibility, mild reaction conditions and was suitable for late-stage modification of drug molecules. Moreover, a preliminary mechanistic probe has been conducted and an exploratory reaction mechanism was proposed. This report represents the first example of an indazolone-directed Cp*Ir(III)-catalyzed C(sp2)–H activation reaction under solvent-free conditions, as well as the first protocol of a Cp*Rh(III)-catalyzed C(sp2)–H activation/oxidation/annulation reaction.
中文翻译:

级联吲唑酮导向的 Ir(III)- 和 Rh(III)- 催化 C(sp2)-H 官能化/[4 + 2] 环化 1-芳基吲唑啉与氧化鎓叶立德,以获得化学上不同的 8H-吲唑 [1,2- a]肉桂啉
通过级联Cp *Ir( III ) -和 Cp*Rh( III )-催化的1 -芳基吲唑酮与锍叶立德。该策略具有底物范围广、官能团相容性高、反应条件温和等特点,适用于药物分子的后期修饰。此外,还进行了初步的机理探索,并提出了探索性反应机理。该报告代表了吲唑酮定向 Cp*Ir( III ) 催化的 C(sp 2)–H 无溶剂条件下的活化反应,以及 Cp*Rh( III ) 催化的 C(sp 2 )–H 活化/氧化/环化反应的第一个方案。
更新日期:2022-08-19
中文翻译:

级联吲唑酮导向的 Ir(III)- 和 Rh(III)- 催化 C(sp2)-H 官能化/[4 + 2] 环化 1-芳基吲唑啉与氧化鎓叶立德,以获得化学上不同的 8H-吲唑 [1,2- a]肉桂啉
通过级联Cp *Ir( III ) -和 Cp*Rh( III )-催化的1 -芳基吲唑酮与锍叶立德。该策略具有底物范围广、官能团相容性高、反应条件温和等特点,适用于药物分子的后期修饰。此外,还进行了初步的机理探索,并提出了探索性反应机理。该报告代表了吲唑酮定向 Cp*Ir( III ) 催化的 C(sp 2)–H 无溶剂条件下的活化反应,以及 Cp*Rh( III ) 催化的 C(sp 2 )–H 活化/氧化/环化反应的第一个方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号