Journal of Hepatology ( IF 26.8 ) Pub Date : 2022-08-13 , DOI: 10.1016/j.jhep.2022.07.016 Elisabetta Degasperi 1 , Maria Paola Anolli 1 , Sara Colonia Uceda Renteria 2 , Dana Sambarino 1 , Marta Borghi 1 , Riccardo Perbellini 1 , Caroline Scholtes 3 , Floriana Facchetti 1 , Alessandro Loglio 1 , Sara Monico 1 , Mirella Fraquelli 4 , Andrea Costantino 4 , Ferruccio Ceriotti 2 , Fabien Zoulim 3 , Pietro Lampertico 5
|
Background & Aims
Bulevirtide (BLV) has recently been conditionally approved for the treatment of chronic hepatitis delta (CHD) in Europe, but its effectiveness and safety in patients with compensated cirrhosis and clinically significant portal hypertension (CSPH) are unknown.
Methods
Consecutive patients with HDV-related compensated cirrhosis and CSPH who started BLV 2 mg/day were enrolled in this single-center study. Clinical/virological characteristics were collected at baseline, weeks 4, 8 and every 8 weeks thereafter. HDV RNA was quantified by Robogene 2.0 (lower limit of detection 6 IU/ml).
Results
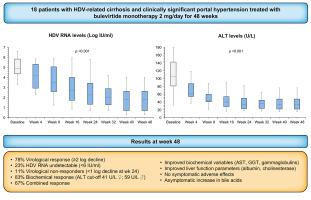
Eighteen Caucasian patients with compensated cirrhosis and CSPH under nucleos(t)ide analogue treatment were enrolled: median (IQR) age was 48 (29-77) years, and 67% were male. Median (IQR) platelet count was 70 (37-227) x103/μl, liver stiffness measurement (LSM) 16.4 (7.8-57.8) kPa, alanine aminotransferase (ALT) 106 (32-222) U/L, HBsAg 3.7 (2.5-4.3) log IU/ml, HDV RNA 4.9 (3.3-6.6) log IU/ml. During 48 weeks of BLV monotherapy, HDV RNA declined by 3.1 (0.2-4.3) log IU/ml (p <0.001 vs. baseline), becoming undetectable in 5 patients (23%). A virological response was observed in 14 (78%) patients while a non-response was observed in 2 (11%). ALT decreased to 35 (15-86) U/L (p <0.001 vs. baseline), normalizing in 83% of patients. A combined response was observed in 67% of patients. Aspartate aminotransferase and gamma-glutamyltransferase levels significantly improved. Concerning liver function parameters, albumin values significantly increased and bilirubin remained stable. LSM significantly improved in patients with virological response, while platelet count was unchanged. None of the patients developed decompensating events or hepatocellular carcinoma. BLV was well tolerated, no patient discontinued treatment and the increase in bile acids was fully asymptomatic.
Conclusions
A 48-week course of BLV 2 mg/day monotherapy is safe and effective even for difficult-to treat patients with HDV-related compensated cirrhosis and CSPH.
Lay summary
Hepatitis delta virus (HDV) is associated with the most severe form of viral hepatitis. A new treatment for HDV called bulevirtide has recently received conditional approval for patients with chronic HDV infection. However, its safety and effectiveness in patients with more advanced liver disease is not known. Herein, we show that it is safe and effective in patients with HDV-related cirrhosis and clinically significant portal hypertension.
中文翻译:

Bulevirtide 单药治疗 HDV 相关代偿性肝硬化和临床显着门静脉高压症患者 48 周
背景与目标
Bulevirtide (BLV) 最近在欧洲被有条件地批准用于治疗慢性丁型肝炎 (CHD),但其在代偿性肝硬化和有临床意义的门静脉高压症 (CSPH) 患者中的有效性和安全性尚不清楚。
方法
开始 BLV 2 mg/天的 HDV 相关代偿性肝硬化和 CSPH 连续患者被纳入这项单中心研究。在基线、第 4 周、第 8 周和此后每 8 周收集临床/病毒学特征。HDV RNA 由 Robogene 2.0 定量(检测下限 6 IU/ml)。
结果
18 名接受核苷(酸)类似物治疗的代偿期肝硬化和 CSPH 白种人患者入选:中位 (IQR) 年龄为 48 (29-77) 岁,67% 为男性。中位 (IQR) 血小板计数为 70 (37-227) x10 3 /μl,肝硬度测量 (LSM) 16.4 (7.8-57.8) kPa,谷丙转氨酶 (ALT) 106 (32-222) U/L, HBsAg 3.7 ( 2.5-4.3) log IU/ml,HDV RNA 4.9 (3.3-6.6) log IU/ml。在 BLV 单一疗法的 48 周期间,HDV RNA 下降了 3.1 (0.2-4.3) log IU/ml(与基线相比p < 0.001 ),在 5 名患者 (23%) 中检测不到。在 14 名 (78%) 患者中观察到病毒学反应,而在 2 名 (11%) 患者中观察到无反应。ALT 降至 35 (15-86) U/L(p < 0.001对比基线),83% 的患者恢复正常。在 67% 的患者中观察到联合反应。天冬氨酸氨基转移酶和γ-谷氨酰转移酶水平显着提高。关于肝功能参数,白蛋白值显着增加,胆红素保持稳定。LSM患者的病毒学反应显着改善,而血小板计数没有变化。没有患者发生失代偿事件或肝细胞癌。BLV 耐受性良好,没有患者停止治疗,胆汁酸的增加完全没有症状。
结论
即使对于难以治疗的 HDV 相关代偿期肝硬化和 CSPH 患者,为期 48 周的 BLV 2 mg/天单一疗法也是安全有效的。
外行总结
丁型肝炎病毒 (HDV) 与最严重的病毒性肝炎有关。一种名为 bulevirtide 的 HDV 新疗法最近获得了有条件的批准,可用于慢性 HDV 感染患者。然而,其在晚期肝病患者中的安全性和有效性尚不清楚。在此,我们证明它对 HDV 相关肝硬化和临床显着门静脉高压症患者安全有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号