Water Research ( IF 11.4 ) Pub Date : 2022-08-14 , DOI: 10.1016/j.watres.2022.118984 Tero Luukkonen 1 , Urs von Gunten 2
|
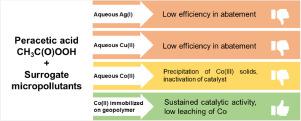
Peracetic acid (PAA) in combination with transition metals has recently gained increasing attention for organic micropollutant abatement. In this study, aqueous Co(II), Cu(II), and Ag(I) were compared for their capacity to activate PAA. Co(II) outperformed Cu(II) or Ag(I) and the optimum conditions were 0.05 mM of Co(II), 0.4 mM of PAA, and pH 3. However, due to a wider applicability in water treatment, pH 7 (i.e., bicarbonate buffer) was selected for detailed investigations. The abatement of different micropollutant surrogates could be described with a second-order rate equation (observed second-order rate constants, kobs were in the range of 42–132 M−1 s−1). For the para-substituted phenols, there was a correlation between the observed second-order rate constants of the corresponding phenolates and the Hammett constants (R2 = 0.949). In all oxidation experiments, the reaction rate decreased significantly after 1–2 min, which coincided with the depletion of PAA but also with the deactivation of the Co(II) catalyst by oxidation to Co(III) and subsequent precipitation. It was demonstrated that Co(II) immobilized on a geopolymer-foam performed approximately similarly as aqueous Co(II) but without deactivation due to Co(III) precipitation. This provides a potential option for the further development of heterogeneous catalytic Co(II)/PAA advanced oxidation processes utilizing geopolymers as a catalyst support material.
中文翻译:

用 Co(II)、Cu(II) 或 Ag(I) 水溶液和地质聚合物负载的 Co(II) 活化的过乙酸氧化有机微污染物替代官能团
最近,过氧乙酸 (PAA) 与过渡金属的结合在减少有机微污染物方面引起了越来越多的关注。在这项研究中,比较了水性 Co(II)、Cu(II) 和 Ag(I) 激活 PAA 的能力。Co(II) 优于 Cu(II) 或 Ag(I),最佳条件是 0.05 mM Co(II)、0.4 mM PAA 和 pH 3。然而,由于在水处理中的更广泛的适用性,pH 7 (即碳酸氢盐缓冲液)被选中进行详细调查。不同微污染物替代物的减少可以用二阶速率方程来描述(观察到的二阶速率常数,k obs在 42-132 M -1 s -1的范围内)。对于第-取代酚,观察到的相应酚盐的二级速率常数和哈米特常数之间存在相关性(R 2 = 0.949)。在所有的氧化实验中,反应速率在 1-2 分钟后显着下降,这与 PAA 的消耗同时发生,同时也与 Co(II) 催化剂通过氧化成 Co(III) 和随后的沉淀失活一致。结果表明,固定在地质聚合物泡沫上的 Co(II) 的性能与 Co(II) 水溶液大致相似,但不会因 Co(III) 沉淀而失活。这为进一步开发利用地质聚合物作为催化剂载体材料的多相催化 Co(II)/PAA 高级氧化工艺提供了潜在的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号