Gastroenterology ( IF 25.7 ) Pub Date : 2022-08-11 , DOI: 10.1053/j.gastro.2022.07.081 Keiko Ishikawa 1 , Shinya Sugimoto 1 , Mayumi Oda 2 , Masayuki Fujii 2 , Sirirat Takahashi 2 , Yuki Ohta 2 , Ai Takano 2 , Kazuhiro Ishimaru 2 , Mami Matano 2 , Kosuke Yoshida 1 , Hikaru Hanyu 2 , Kohta Toshimitsu 2 , Kazuaki Sawada 3 , Mariko Shimokawa 2 , Megumu Saito 4 , Kenta Kawasaki 1 , Ryota Ishii 5 , Koji Taniguchi 6 , Takeshi Imamura 7 , Takanori Kanai 8 , Toshiro Sato 2
|
Background & Aims
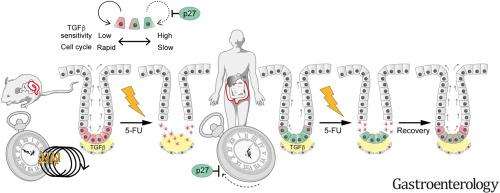
In the mouse intestinal epithelium, Lgr5+ stem cells are vulnerable to injury, owing to their predominantly cycling nature, and their progenies de-differentiate to replenish the stem cell pool. However, how human colonic stem cells behave in homeostasis and during regeneration remains unknown.
Methods
Transcriptional heterogeneity among colonic epithelial cells was analyzed by means of single-cell RNA sequencing analysis of human and mouse colonic epithelial cells. To trace the fate of human colonic stem or differentiated cells, we generated LGR5-tdTomato, LGR5-iCasase9-tdTomato, LGR5-split-Cre, and KRT20-ERCreER knock-in human colon organoids via genome engineering. p27+ dormant cells were further visualized with the p27-mVenus reporter. To analyze the dynamics of human colonic stem cells in vivo, we orthotopically xenotransplanted fluorescence-labeled human colon organoids into immune-deficient mice. The cell cycle dynamics in xenograft cells were evaluated using 5-ethynyl-2′-deoxyuridine pulse-chase analysis. The clonogenic capacity of slow-cycling human stem cells or differentiated cells was analyzed in the context of homeostasis, LGR5 ablation, and 5-fluorouracil–induced mucosal injury.
Results
Single-cell RNA sequencing analysis illuminated the presence of nondividing LGR5+ stem cells in the human colon. Visualization and lineage tracing of slow-cycling LGR5+p27+ cells and orthotopic xenotransplantation validated their homeostatic lineage-forming capability in vivo, which was augmented by 5-FU–induced mucosal damage. Transforming growth factor–β signaling regulated the quiescent state of LGR5+ cells. Despite the plasticity of differentiated KRT20+ cells, they did not display clonal growth after 5-FU–induced injury, suggesting that occupation of the niche environment by LGR5+p27+ cells prevented neighboring differentiated cells from de-differentiating.
Conclusions
Our results highlight the quiescent nature of human LGR5+ colonic stem cells and their contribution to post-injury regeneration.
中文翻译:

鉴定人结肠中静止的 LGR5+ 干细胞
背景与目标
在小鼠肠上皮中,Lgr5 +干细胞由于其主要的循环性质而容易受到损伤,并且它们的后代去分化以补充干细胞库。然而,人类结肠干细胞在体内平衡和再生过程中的表现仍然未知。
方法
通过人和小鼠结肠上皮细胞的单细胞 RNA 测序分析,分析结肠上皮细胞之间的转录异质性。为了追踪人类结肠干细胞或分化细胞的命运,我们通过基因组工程生成了 LGR5-tdTomato、LGR5-iCasase9-tdTomato、LGR5-split-Cre 和 KRT20-ERCreER 敲入人类结肠类器官。p27 +使用 p27-mVenus 报告基因进一步观察休眠细胞。为了分析人结肠干细胞在体内的动态,我们将荧光标记的人结肠类器官原位异种移植到免疫缺陷小鼠体内。使用 5-乙炔基-2'-脱氧尿苷脉冲追踪分析评估异种移植细胞中的细胞周期动力学。在稳态、LGR5 消融和 5-氟尿嘧啶诱导的粘膜损伤的背景下分析了慢循环人类干细胞或分化细胞的克隆能力。
结果
单细胞 RNA 测序分析阐明了人类结肠中存在不分裂的 LGR5 +干细胞。慢循环 LGR5 + p27 +细胞和原位异种移植的可视化和谱系追踪验证了它们在体内的稳态谱系形成能力,5-FU 诱导的粘膜损伤增强了这种能力。转化生长因子-β 信号调节 LGR5 +细胞的静止状态。尽管分化的 KRT20 +细胞具有可塑性,但它们在 5-FU 诱导的损伤后没有表现出克隆生长,这表明 LGR5 + p27 +占据了生态位环境。细胞阻止邻近的分化细胞去分化。
结论
我们的研究结果突出了人类 LGR5 +结肠干细胞的静止性质及其对损伤后再生的贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号