Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2022-08-11 , DOI: 10.1016/j.jclepro.2022.133569 Shunan Yin , Jinxian Zhao , Shiping Wu , Xuhui Wang , Yanhong Quan , Jun Ren
|
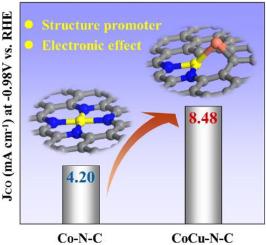
Electrochemical reduction of CO2 to valuable chemicals using electricity from renewable sources is a promising approach to realize the carbon cycle. Here, a novel bimetallic nitrogen-doped carbon catalyst (M-N-C, where M denotes the metal) CoCu–N–C electrocatalyst has been synthesized by pyrolyzing a copper-doped cobalt(II) acetate–phenanthroline complex formed from the metal acetates and 1,10-phenanthroline dissolved in ethanol. The optimal CoCu–N–C catalyst with molar ratio of Cu/(Co + Cu) = 0.15 displayed superior performance, enabling a faradaic efficiency of CO production of 76.5% at an overpotential of 0.57 V as well as an increased CO current density of 8.48 mA cm−2 at −0.98 V versus reversible hydrogen electrode (far superior than the Co–N–C of 4.20 mA cm−2 and Cu–N–C of 0.07 mA cm−2) and a decreased charge-transfer resistance compared with Co–N–C and Cu–N–C catalysts. The addition of Cu, which serves as a structure promoter to improve the dispersion of Co, thereby providing sufficient Co-Nx active sites for the electrochemical CO2 reduction reaction (CRR). Moreover, doping Cu changes the electronic environment around Co slightly and promotes the charge transfer capability so that reduces the energy barrier of potential-limiting energy, thereby improving the CRR performance of catalyst.
中文翻译:

在双金属 CoCu-N-C 催化剂上将 CO2 电化学还原为 CO
使用来自可再生能源的电力将 CO 2电化学还原为有价值的化学品是实现碳循环的有前景的方法。在这里,一种新型双金属氮掺杂碳催化剂(MNC,其中 M 表示金属)CoCu-N-C电催化剂是通过热解由金属乙酸盐和 1 形成的铜掺杂的乙酸钴 (II)-菲咯啉配合物合成的, 10-菲咯啉溶于乙醇。摩尔比为 Cu/(Co + Cu) = 0.15 的最佳 CoCu-N-C 催化剂表现出优异的性能,在 0.57 V 的过电位下,CO 的法拉第效率为 76.5%,CO 电流密度为8.48毫安厘米-2在 -0.98 V 时与可逆氢电极相比(远优于 4.20 mA cm -2的 Co -N-C 和 0.07 mA cm -2的 Cu-N-C ),并且与 Co-N- 相比,电荷转移电阻降低C和Cu-N-C催化剂。添加Cu作为结构促进剂以改善Co的分散,从而为电化学CO 2还原反应(CRR)提供足够的Co-N x活性位点。此外,掺杂Cu略微改变了Co周围的电子环境,提高了电荷转移能力,从而降低了限位能的能垒,从而提高了催化剂的CRR性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号