Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2022-08-10 , DOI: 10.1016/j.jhazmat.2022.129760 Liang Wu 1 , Samuel D Patton 1 , Haizhou Liu 1
|
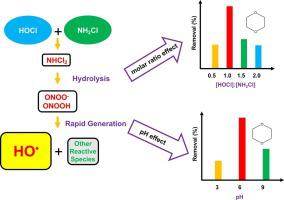
Free chlorine (HOCl) and monochloramine (NH2Cl) are two of the most commonly used water disinfectants in water treatment; however, the capability of rapid mixing of HOCl into NH2Cl to induce oxidative reactions for efficient removal of contaminants remains largely unknown. In this study, 1,4-dioxane (1,4-D) removal was quantified during the rapid mixing of HOCl into NH2Cl, to evaluate the effects of solution pH and HOCl-to-NH2Cl ratio, and to identify mechanisms by which reactive species are generated in the system. Results showed that the highest 1,4-D removal was observed at the near-neutral pH of 6 with the HOCl-to-NH2Cl molar ratio of 1. Hydroxyl radical (HO•) contributed to 60–70 % of 1,4-D degradation and its generation was initiated by the hydrolytic decay of NH2Cl and NHCl2 upon HOCl addition to NH2Cl with rapid mixing, and subsequent transformation of peroxynitrite (ONOO-) and peroxynitrous acid (ONOOH). The results also confirmed that the presence of dissolved oxygen was required to form ONOO-/ONOOH, and ONOO- was a crucial precursor for reactive radical generation. These findings provide insight into the reaction mechanism associated with the system of rapidly mixed HOCl into NH2Cl with the potential optimization and application for efficient trace organics removal in water treatment and reuse.
中文翻译:

游离氯快速混合成一氯胺氧化去除 1,4-二恶烷的机理:对水处理和再利用的影响
游离氯(HOCl)和一氯胺(NH 2 Cl)是水处理中最常用的两种水消毒剂;然而,HOCl 快速混合到 NH 2 Cl 中以诱导氧化反应以有效去除污染物的能力仍然很大程度上未知。在本研究中,量化了 HOCl 与 NH 2 Cl 快速混合过程中 1,4-二恶烷 (1,4-D) 的去除情况,以评估溶液 pH 值和 HOCl 与 NH 2 Cl 比的影响,并确定在系统中产生活性物质的机制。结果表明,在接近中性的 pH 值为 6 且 HOCl 与 NH 2 Cl 的摩尔比为 1 时,1,4-D 的去除率最高。)促成了 60-70% 的 1,4- D降解,其生成是由 NH 2 Cl和NHCl 2的水解衰变引发的和过氧亚硝酸(ONOOH)。结果还证实,溶解氧的存在是形成 ONOO - /ONOOH 所必需的,而 ONOO -是反应性自由基产生的关键前体。这些发现为深入了解与快速混合 HOCl 到 NH 2 Cl 的系统相关的反应机制提供了潜在的优化和应用,以在水处理和再利用中有效去除痕量有机物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号