当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultra-low voltage bipolar hydrogen production from biomass-derived aldehydes and water in membrane-less electrolyzers
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2ee01427k Hengzhou Liu 1 , Naveen Agrawal 2 , Arna Ganguly 3 , Yifu Chen 1 , Jungkuk Lee 1 , Jiaqi Yu 4 , Wenyu Huang 4 , Mark Mba Wright 3 , Michael J. Janik 2 , Wenzhen Li 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2ee01427k Hengzhou Liu 1 , Naveen Agrawal 2 , Arna Ganguly 3 , Yifu Chen 1 , Jungkuk Lee 1 , Jiaqi Yu 4 , Wenyu Huang 4 , Mark Mba Wright 3 , Michael J. Janik 2 , Wenzhen Li 1
Affiliation
|
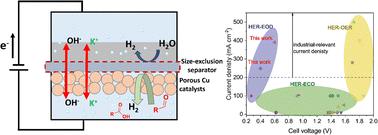
Water electrolysis using renewable energy inputs is being actively pursued as a green route for hydrogen production. However, it is limited by the high energy consumption due to the sluggish anodic oxygen evolution reaction (OER) and safety issues associated with H2 and O2 mixing. Here, we replaced the OER with an electrocatalytic oxidative dehydrogenation (EOD) of aldehydes for bipolar H2 production and achieved industrial-level current densities at cell voltages much lower than during water electrolysis. Experimental and computational studies suggest a reasonable barrier for C–H dissociation on Cu surfaces, mainly through a diol intermediate, with a potential-dependent competition with the solution-phase Cannizzaro reaction. The kinetics of the EOD reaction was further enhanced using a porous CuAg catalyst prepared from a galvanic replacement method. Through Ag incorporation and its modification on the Cu surface, the geometric current density and electrocatalyst durability were significantly improved. Finally, we engineered a bipolar H2 production system in membrane–electrode assembly-based flow cells to facilitate mass transport, achieving maximum current densities of 248 and 390 mA cm−2 at cell voltages of 0.4 V and 0.6 V, respectively. The faradaic efficiency of H2 from both the cathode and anode reactions attained ∼100%. Taking advantage of the bipolar H2 production without the issues associated with H2/O2 mixing, an inexpensive, easy-to-manufacture dialysis porous membrane was demonstrated to substitute the costly anion exchange membrane, achieving an energy-efficient and cost-effective process in a simple reactor for H2 production. An estimated H2 price of $2.51/kg from an initial technoeconomic assessment is competitive with US DoE's “Green H2” targets.
中文翻译:

在无膜电解槽中由生物质衍生的醛和水进行超低压双极制氢
作为制氢的绿色途径,正在积极寻求使用可再生能源输入的水电解。然而,由于缓慢的阳极析氧反应(OER)和与H 2和O 2混合相关的安全问题,它受到高能耗的限制。在这里,我们用醛的电催化氧化脱氢 (EOD) 代替了 OER,用于双极性 H 2生产并在电池电压远低于水电解期间实现了工业水平的电流密度。实验和计算研究表明,铜表面上的 C-H 解离存在合理的障碍,主要通过二醇中间体,与溶液相 Cannizzaro 反应具有电位依赖性竞争。使用由电置换法制备的多孔 CuAg 催化剂进一步增强了 EOD 反应的动力学。通过在Cu表面掺入Ag并对其进行改性,几何电流密度和电催化剂耐久性显着提高。最后,我们在基于膜电极组件的流通池中设计了一个双极 H 2生产系统,以促进质量传输,实现 248 和 390 mA cm 的最大电流密度-2在电池电压分别为 0.4 V 和 0.6 V 时。来自阴极和阳极反应的H 2的法拉第效率均达到~100%。利用双极 H 2生产而没有与 H 2 /O 2混合相关的问题,一种廉价、易于制造的透析多孔膜被证明可以替代昂贵的阴离子交换膜,实现了节能和成本效益在一个简单的反应器中生产 H 2的过程。根据初步技术经济评估,估计 H 2价格为 2.51 美元/公斤,与美国能源部的“绿色 H 2 ”目标具有竞争力。
更新日期:2022-08-12
中文翻译:

在无膜电解槽中由生物质衍生的醛和水进行超低压双极制氢
作为制氢的绿色途径,正在积极寻求使用可再生能源输入的水电解。然而,由于缓慢的阳极析氧反应(OER)和与H 2和O 2混合相关的安全问题,它受到高能耗的限制。在这里,我们用醛的电催化氧化脱氢 (EOD) 代替了 OER,用于双极性 H 2生产并在电池电压远低于水电解期间实现了工业水平的电流密度。实验和计算研究表明,铜表面上的 C-H 解离存在合理的障碍,主要通过二醇中间体,与溶液相 Cannizzaro 反应具有电位依赖性竞争。使用由电置换法制备的多孔 CuAg 催化剂进一步增强了 EOD 反应的动力学。通过在Cu表面掺入Ag并对其进行改性,几何电流密度和电催化剂耐久性显着提高。最后,我们在基于膜电极组件的流通池中设计了一个双极 H 2生产系统,以促进质量传输,实现 248 和 390 mA cm 的最大电流密度-2在电池电压分别为 0.4 V 和 0.6 V 时。来自阴极和阳极反应的H 2的法拉第效率均达到~100%。利用双极 H 2生产而没有与 H 2 /O 2混合相关的问题,一种廉价、易于制造的透析多孔膜被证明可以替代昂贵的阴离子交换膜,实现了节能和成本效益在一个简单的反应器中生产 H 2的过程。根据初步技术经济评估,估计 H 2价格为 2.51 美元/公斤,与美国能源部的“绿色 H 2 ”目标具有竞争力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号