当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed arylative substitution of homoallylic alcohols
Chemical Science ( IF 8.4 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2sc01716d Hai N Tran 1 , Chau M Nguyen 1 , Mason T Koeritz 1 , Dustin D Youmans 1 , Levi M Stanley 1
Chemical Science ( IF 8.4 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2sc01716d Hai N Tran 1 , Chau M Nguyen 1 , Mason T Koeritz 1 , Dustin D Youmans 1 , Levi M Stanley 1
Affiliation
|
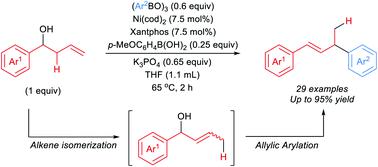
Direct coupling of unactivated alcohols remains a challenge in synthetic chemistry. Current approaches to cross-coupling of alcohol-derived electrophiles often involve activated alcohols such as tosylates or carbonates. We report the direct arylative substitution of homoallylic alcohols catalyzed by a nickel-bisphosphine complex as a facile method to generate allylic arenes. These reactions proceed via formation of an allylic alcohol intermediate. Subsequent allylic substitution with arylboroxine nucleophiles enables the formation of a variety of allylic arenes. The presence of p-methoxyphenylboronic acid is crucial to activate the allylic alcohol to achieve high product yields.
中文翻译:

镍催化的高烯丙醇芳基取代
未活化醇的直接偶联仍然是合成化学中的一个挑战。当前对醇衍生的亲电子试剂进行交叉偶联的方法通常涉及活化的醇,例如甲苯磺酸盐或碳酸盐。我们报告了由镍-双膦配合物催化的高烯丙醇的直接芳基取代作为一种简便的方法来生成烯丙基芳烃。这些反应通过形成烯丙醇中间体进行。随后用芳基环硼氧烷亲核试剂进行烯丙基取代能够形成多种烯丙基芳烃。对甲氧基苯基硼酸的存在对于活化烯丙醇以实现高产率至关重要。
更新日期:2022-08-12
中文翻译:

镍催化的高烯丙醇芳基取代
未活化醇的直接偶联仍然是合成化学中的一个挑战。当前对醇衍生的亲电子试剂进行交叉偶联的方法通常涉及活化的醇,例如甲苯磺酸盐或碳酸盐。我们报告了由镍-双膦配合物催化的高烯丙醇的直接芳基取代作为一种简便的方法来生成烯丙基芳烃。这些反应通过形成烯丙醇中间体进行。随后用芳基环硼氧烷亲核试剂进行烯丙基取代能够形成多种烯丙基芳烃。对甲氧基苯基硼酸的存在对于活化烯丙醇以实现高产率至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号