当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling hydridic-to-protonic dihydrogen bond predominance in monohydrated dodecaborate clusters
Chemical Science ( IF 7.6 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2sc03986a Yanrong Jiang 1 , Qinqin Yuan 2, 3 , Wenjin Cao 2 , Zhubin Hu 1 , Yan Yang 1 , Cheng Zhong 4 , Tao Yang 1 , Haitao Sun 1, 5 , Xue-Bin Wang 2 , Zhenrong Sun 1, 5
Chemical Science ( IF 7.6 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2sc03986a Yanrong Jiang 1 , Qinqin Yuan 2, 3 , Wenjin Cao 2 , Zhubin Hu 1 , Yan Yang 1 , Cheng Zhong 4 , Tao Yang 1 , Haitao Sun 1, 5 , Xue-Bin Wang 2 , Zhenrong Sun 1, 5
Affiliation
|
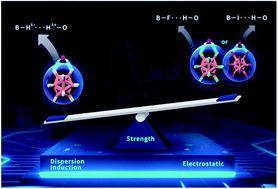
Hydridic-to-protonic dihydrogen bonds (DHBs) are involved in comprehensive structural and energetic evolution, and significantly affect reactivity and selectivity in solution and solid states. Grand challenges exist in understanding DHBs’ bonding nature and strength, and how to harness DHBs. Herein we launched a combined photoelectron spectroscopy and multiscale theoretical investigation using monohydrated closo-dodecaborate clusters B12X122−·H2O (X = H, F, I) to address such challenges. For the first time, a consistent and unambiguous picture is unraveled demonstrating that B–H⋯H–O DHBs are superior to the conventional B–X⋯H–O HBs, being 1.15 and 4.61 kcal mol−1 stronger than those with X = F and I, respectively. Energy decomposition analyses reveal that induction and dispersion terms make pronounced contributions resulting in a stronger B–H⋯H–O DHB. These findings call out more attention to the prominent roles of DHBs in water environments and pave the way for efficient and eco-friendly catalytic dihydrogen production based on optimized hydridic-to-protonic interactions.
中文翻译:

揭示一水十二硼酸盐簇中氢对质子二氢键的优势
氢质子二氢键(DHB)参与全面的结构和能量演化,并显着影响溶液和固态的反应性和选择性。了解 DHB 的结合性质和强度以及如何利用 DHB 存在巨大的挑战。在此,我们利用一水合近十二硼酸盐簇 B 12 X 12 2− ·H 2 O (X = H, F, I) 开展了光电子能谱和多尺度理论研究相结合来应对这些挑战。首次揭示了一致且明确的图片,证明 B–H⋯H–O DHB 优于传统的 B–X⋯H–O HB,比 X = 的那些强 1.15 和 4.61 kcal mol −1分别为F和I。能量分解分析表明,感应项和色散项做出了显着的贡献,导致了更强的 B–H⋯H–O DHB。这些发现唤起了人们对 DHB 在水环境中的突出作用的更多关注,并为基于优化的氢与质子相互作用的高效且环保的催化产氢铺平了道路。
更新日期:2022-08-12
中文翻译:

揭示一水十二硼酸盐簇中氢对质子二氢键的优势
氢质子二氢键(DHB)参与全面的结构和能量演化,并显着影响溶液和固态的反应性和选择性。了解 DHB 的结合性质和强度以及如何利用 DHB 存在巨大的挑战。在此,我们利用一水合近十二硼酸盐簇 B 12 X 12 2− ·H 2 O (X = H, F, I) 开展了光电子能谱和多尺度理论研究相结合来应对这些挑战。首次揭示了一致且明确的图片,证明 B–H⋯H–O DHB 优于传统的 B–X⋯H–O HB,比 X = 的那些强 1.15 和 4.61 kcal mol −1分别为F和I。能量分解分析表明,感应项和色散项做出了显着的贡献,导致了更强的 B–H⋯H–O DHB。这些发现唤起了人们对 DHB 在水环境中的突出作用的更多关注,并为基于优化的氢与质子相互作用的高效且环保的催化产氢铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号