当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric organocatalytic sulfenylation for the construction of a diheteroatom-bearing tetrasubstituted carbon centre
Chemical Communications ( IF 4.3 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2cc03443c Qingfa Tan 1 , Qianping Chen 1 , Zitong Zhu 1 , Xiaohua Liu 1
Chemical Communications ( IF 4.3 ) Pub Date : 2022-08-12 , DOI: 10.1039/d2cc03443c Qingfa Tan 1 , Qianping Chen 1 , Zitong Zhu 1 , Xiaohua Liu 1
Affiliation
|
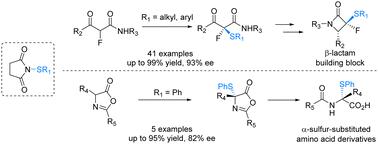
Catalytic enantioselective sulfenylation to construct diheteroatom-bearing carbon centres was achieved by employing chiral guanidine organocatalysts. This protocol provided a facile route towards the synthesis of α-fluoro-α-sulfenyl-β-ketoamides, azlactone adducts and α-sulfur-substituted amino acid derivatives in high yields with good to excellent enantioselectivities. A possible working mode was proposed to elucidate the chiral control of the process.
中文翻译:

不对称有机催化亚磺酰化构建含二杂原子的四取代碳中心
通过使用手性胍有机催化剂,实现了催化对映选择性亚磺酰化构建含二杂原子的碳中心。该协议为以高产率合成 α-氟-α-硫基-β-酮酰胺、azlactone 加合物和 α-硫取代的氨基酸衍生物提供了一条简便的途径,具有良好的对映选择性。提出了一种可能的工作模式来阐明该过程的手性控制。
更新日期:2022-08-12
中文翻译:

不对称有机催化亚磺酰化构建含二杂原子的四取代碳中心
通过使用手性胍有机催化剂,实现了催化对映选择性亚磺酰化构建含二杂原子的碳中心。该协议为以高产率合成 α-氟-α-硫基-β-酮酰胺、azlactone 加合物和 α-硫取代的氨基酸衍生物提供了一条简便的途径,具有良好的对映选择性。提出了一种可能的工作模式来阐明该过程的手性控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号