Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2022-08-11 , DOI: 10.1016/j.jclepro.2022.133526 Xuan Guo , Lin Zhu , Xin Xu , Maoting Ma , Guoyuan Zou , Dan Wei
|
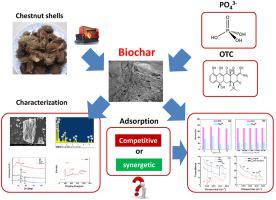
In the present study, the potential competitive or synergetic adsorption mechanism of phosphate and antibiotics from a solution using chestnut shell (CS)-derived biochar was explored. CSs were utilized as the raw material to prepare biochar (BCCS) and modified biochar (MBCCS) via an oxygen-isolated pyrolysis, and subsequent modification using KMnO4 and FeCl3. Following the carbonization, the biochar was deoxidized, which enhanced its porous structure and specific surface area. The MBCCS, which contained Fe and Mn ions on its surfaces, showed theoretical adsorption capacities of 0.7880 mg/g P and 6.2854 mg/g oxytetracycline (OTC), respectively. The adsorption of both PO43− and OTC by the MBCCS fitted better to the pseudo-second-order kinetic model. The PO43− adsorption on the MBCCS fitted better to the Langmuir isotherm, whereas that of the OTC was adequately described via the Freundlich isotherm. The adsorption processes were both endothermic. The pH and cation strength of the solution significantly affected the adsorption of both PO43− and OTC. Low concentrations of OTC favored competitive adsorption with PO43−, while high concentrations of both components produced synergetic adsorption on the MBCCS. In the present study, a potential method with good reusability for the utilization of agricultural and forestry wastes is highlighted. The results also provide a scientific basis for elucidating the adsorption mechanisms of coexisting pollutants in water on biochar.











































 京公网安备 11010802027423号
京公网安备 11010802027423号