Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-08-10 , DOI: 10.1016/j.apcatb.2022.121821 Paolo A. Cuello-Penaloza , Javier Chavarrio-Cañas , Yi Du , Michael P. Lanci , Derek A. Maedke , James A. Dumesic , George W. Huber
|
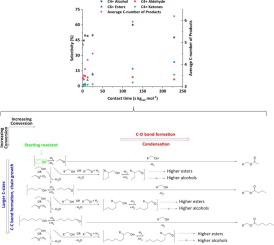
We study ethanol oligomerization to higher alcohols and other oxygenates with a 0.3 wt. %Cu/Mg2.9AlO catalyst. This reaction involves more than 130 products in a complicated reaction network. The selectivity towards diesel fuel precursor compounds (hereafter ‘DFPC’) increased with conversion until reaching a plateau at an ethanol conversion of ~70 %. Alcohol selectivity was found to follow Schultz-Flory distribution at all studied conversions. Larger sized alcohols then are formed mostly by chain-growth mechanisms via surface reactions of adsorbed oligomers with ethanol-based monomers. Higher esters are formed from alcohols and aldehydes in a series reaction mechanism. Moreover, C6+ ester and C4+ ketones selectivities increase as conversion increases. We also found that C4+ alcohols most likely undergo Guerbet coupling with the studied catalyst to form even higher alcohols once, and that the oxygen of these alcohols is active as a nucleophile, resulting in the selective formation of esters if the starting alcohol is branched. Finally, we performed several cofeed studies varying ethanol-to-H2 inlet partial pressures, and adding acetaldehyde and ethyl acetate as cofeeds to ethanol at different concentrations. We conclude from these experiments that acetaldehyde concentration controls reaction chemistry, with conditions favoring larger concentrations of the molecule promoting both alcohol coupling and ester formation, and conditions leading to lower concentrations of acetaldehyde resulting in higher alcohol selectivity at the expense of esters and higher aldehydes.
中文翻译:

使用低负载 Cu/MgxAlOy 催化剂的乙醇低聚生成馏分分子的反应化学
我们用 0.3 wt. 研究乙醇低聚成高级醇和其他含氧化合物。%Cu/Mg 2.9 Al2O 催化剂。该反应涉及复杂反应网络中的 130 多种产物。对柴油燃料前体化合物(以下简称“DFPC”)的选择性随着转化率的增加而增加,直到乙醇转化率达到约 70% 的平台期。在所有研究的转化率中,发现酒精选择性遵循 Schultz-Flory 分布。然后,较大尺寸的醇主要通过吸附的低聚物与乙醇基单体的表面反应通过链增长机制形成。高级酯是由醇和醛以串联反应机理形成的。此外,C 6+酯和 C 4+酮的选择性随着转化率的增加而增加。我们还发现,C 4+醇最有可能与所研究的催化剂发生 Guerbet 偶联以形成更高级的醇,并且这些醇的氧作为亲核试剂具有活性,如果起始醇是支链的,则导致选择性形成酯. 最后,我们进行了几项将乙醇转化为 H 2的共进料研究入口分压,并将乙醛和乙酸乙酯作为共同进料添加到不同浓度的乙醇中。我们从这些实验中得出结论,乙醛浓度控制反应化学,有利于较大浓度的分子促进醇偶联和酯形成的条件,以及导致较低浓度的乙醛导致较高的醇选择性而牺牲酯和较高醛的条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号