当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Marine Alkaloids Cystodytins A–K
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-10 , DOI: 10.1021/acs.joc.2c01317 Dongfang Jiang 1 , Yang Chen 1 , Shaozhong Wang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-10 , DOI: 10.1021/acs.joc.2c01317 Dongfang Jiang 1 , Yang Chen 1 , Shaozhong Wang 1
Affiliation
|
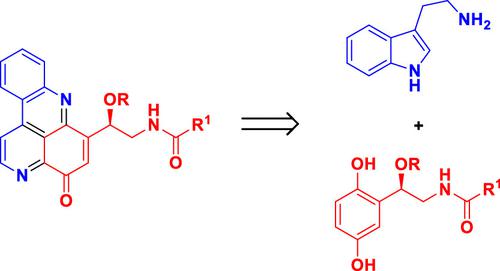
The total synthesis of marine alkaloids cystodytins A–K has been accomplished in five to six steps starting from commercially available compounds. The highlights of the synthesis include an oxidative amination–cyclization of tryptamine and para-hydroquinones to build a tetracyclic pyridoacridinone ring with different side chains and a copper(II)-catalyzed enantioselective Henry reaction to construct an oxygenated stereogenic carbon center. For the first time, the absolute configuration of the stereogenic centers embedded in cystodytins D–I and K was established as R. Moreover, the stereochemistry of the olefin unit in the side chain of cystodytins H and I was revised to the Z configuration from the originally assigned E configuration.
中文翻译:

海洋生物碱 Cystodytins A–K 的全合成
从市售化合物开始,海洋生物碱 cystodytins A-K 的全合成分五到六个步骤完成。合成的亮点包括色胺和对氢醌的氧化胺化环化以构建具有不同侧链的四环吡啶并吖啶酮环和铜(II)催化的对映选择性亨利反应以构建氧化的立体碳中心。首次将囊性素 D-I 和 K 中嵌入的立体中心的绝对构型确定为R。此外,cystodytins H 和 I 侧链中烯烃单元的立体化学从最初指定的E构型修改为Z构型。
更新日期:2022-08-10
中文翻译:

海洋生物碱 Cystodytins A–K 的全合成
从市售化合物开始,海洋生物碱 cystodytins A-K 的全合成分五到六个步骤完成。合成的亮点包括色胺和对氢醌的氧化胺化环化以构建具有不同侧链的四环吡啶并吖啶酮环和铜(II)催化的对映选择性亨利反应以构建氧化的立体碳中心。首次将囊性素 D-I 和 K 中嵌入的立体中心的绝对构型确定为R。此外,cystodytins H 和 I 侧链中烯烃单元的立体化学从最初指定的E构型修改为Z构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号