Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2022-08-08 , DOI: 10.1016/j.jallcom.2022.166706 Wei Liu , Wenyu Tan , Yang Yang , Hanwei He
|
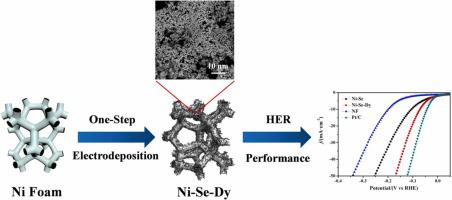
Efficient, stable, and environmentally friendly electrocatalysts urgently need to be explored for hydrogen evolution reaction (HER). A novel and facile nickel–selenium–dysprosium (Ni–Se–Dy) electrocatalyst was synthesized on Ni foam via simple and feasible one–step galvanostatic electrodeposition. The prepared Ni–Se–Dy electrodes showed excellent HER activity in alkaline medium with low overpotential values of 72 and 173 mV to deliver the current density values of 10 and 50 mA·cm−2. Tafel slope value (119.72 mV·dec−1) for Ni–Se–Dy electrode in 1.0 M KOH indicated that the Ni–Se–Dy catalyst followed a Volmer–Heyrovsky mechanism, and the rate–determining step of this reaction was a Volmer step. Electrochemical test and scanning electron microscopy results showed that the excellent HER performance of Ni–Se–Dy electrode can be attributed to surface morphology, better intrinsic activity, and high conductivity.
中文翻译:

Ni-Se-Dy膜在Ni泡沫上的一步恒电流电沉积用于碱性溶液中的析氢反应
析氢反应(HER)急需探索高效、稳定、环保的电催化剂。通过简单可行的一步恒电流电沉积,在泡沫镍上合成了一种新颖易行的镍-硒-镝 (Ni-Se-Dy) 电催化剂。制备的 Ni-Se-Dy 电极在碱性介质中表现出优异的 HER 活性,具有 72 和 173 mV 的低过电位值,可提供 10 和 50 mA·cm -2的电流密度值。Tafel斜率值(119.72 mV·dec -1) 对于 1.0 M KOH 中的 Ni-Se-Dy 电极,表明 Ni-Se-Dy 催化剂遵循 Volmer-Heyrovsky 机理,该反应的速率决定步骤是 Volmer 步骤。电化学测试和扫描电子显微镜结果表明,Ni-Se-Dy电极优异的HER性能可归因于表面形貌、更好的本征活性和高导电性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号