Talanta ( IF 5.6 ) Pub Date : 2022-08-09 , DOI: 10.1016/j.talanta.2022.123780 Julien Camperi 1 , Gary Console 2 , Laura Zheng 2 , Nicole Stephens 2 , Mary Montti 2 , Brian Roper 2 , Minhua Zheng 3 , Maryam Moshref 1 , Yavuz Dagdas 4 , Patrick Holder 4 , Cinzia Stella 2
|
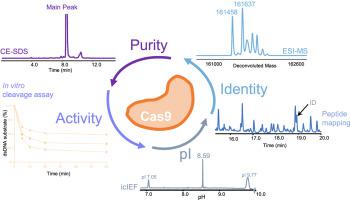
CRISPR (clustered regularly interspaced short palindromic repeats)-associated proteins (Cas) are powerful gene-editing tools used in therapeutic applications. Efforts to minimize off-target cleavage by CRISPR-Cas9 have motivated the development of engineered Cas9 variants. The wild-type (WT) Streptococcus pyogenes (SpCas9) has been engineered into a high-fidelity Cas9 (SpyFi Cas9) that shows promising results in providing high on-target activity (targeting efficiency) while reducing off-target editing (unwanted mutations). This work describes for the first time the development of ultra-high-performance liquid chromatography (UHPLC) and capillary electrophoresis (CE)-based methods for a full characterization of different engineered Cas9 variants, including determination of purity, size variants, isoelectric points (pI), post-translational modifications (PTMs), and functional activities. The purity and size variant characterization were first determined by CE-sodium dodecyl sulfate (SDS). An in vitro DNA cleavage assay using an automated electrophoresis tool was employed to investigate the functional activity of ribonucleoprotein (RNP) complexes derived from Cas9 variants. The pIs of the engineered Cas9 proteins were determined by imaged capillary isoelectric focusing (icIEF), while intact mass measurements were performed by reversed-phase (RP)-UHPLC coupled with high-resolution mass spectrometry (HRMS). A peptide mapping assay based on LC-UV-MS/MS using endoproteinase Lys-C under non-reducing conditions was developed to confirm amino acid sequences, allowing differentiation of SpyFi Cas9 from WT SpCas9. The potential of using a low-resolution MS detector, especially for a GMP environment, as a low-cost and simple method to identify SpyFi Cas9 is discussed.
中文翻译:

基于 UHPLC 和 CE 的综合方法,用于工程化 Cas9 表征
CRISPR(成簇规律间隔的短回文重复序列)相关蛋白 (Cas) 是用于治疗应用的强大基因编辑工具。尽量减少 CRISPR-Cas9 的脱靶切割的努力推动了工程化 Cas9 变体的开发。野生型 (WT)化脓性链球菌(SpCas9) 已被设计成高保真 Cas9 (SpyFi Cas9),在提供高靶向活性(靶向效率)同时减少脱靶编辑(不需要的突变)方面显示出可喜的结果。这项工作首次描述了基于超高效液相色谱 (UHPLC) 和毛细管电泳 (CE) 的方法的发展,以全面表征不同的工程 Cas9 变体,包括纯度、大小变体、等电点的测定( pI)、翻译后修饰 (PTM) 和功能活动。纯度和大小变异特征首先由 CE-十二烷基硫酸钠 (SDS) 确定。体外_使用自动电泳工具进行 DNA 裂解测定,用于研究源自 Cas9 变体的核糖核蛋白 (RNP) 复合物的功能活性。工程化 Cas9 蛋白的 pI 通过成像毛细管等电聚焦 (icIEF) 确定,而完整质量测量通过反相 (RP)-UHPLC 结合高分辨率质谱 (HRMS) 进行。开发了一种基于 LC-UV-MS/MS 在非还原条件下使用内切蛋白酶 Lys-C 的肽图分析法,以确认氨基酸序列,从而可以将 SpyFi Cas9 与 WT SpCas9 区分开来。讨论了使用低分辨率 MS 检测器作为识别 SpyFi Cas9 的低成本和简单方法的潜力,特别是对于 GMP 环境。











































 京公网安备 11010802027423号
京公网安备 11010802027423号