当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Side-Chain Hydrophilicity on Packing, Swelling, and Ion Interactions in Oxy-Bithiophene Semiconductors
Advanced Materials ( IF 27.4 ) Pub Date : 2022-08-09 , DOI: 10.1002/adma.202204258 Nicholas Siemons 1 , Drew Pearce 1 , Camila Cendra 2 , Hang Yu 1 , Sachetan M Tuladhar 1 , Rawad K Hallani 3 , Rajendar Sheelamanthula 3 , Garrett S LeCroy 2 , Lucas Siemons 4 , Andrew J P White 5 , Iain McCulloch 6 , Alberto Salleo 2 , Jarvist M Frost 1 , Alexander Giovannitti 2 , Jenny Nelson 1
Advanced Materials ( IF 27.4 ) Pub Date : 2022-08-09 , DOI: 10.1002/adma.202204258 Nicholas Siemons 1 , Drew Pearce 1 , Camila Cendra 2 , Hang Yu 1 , Sachetan M Tuladhar 1 , Rawad K Hallani 3 , Rajendar Sheelamanthula 3 , Garrett S LeCroy 2 , Lucas Siemons 4 , Andrew J P White 5 , Iain McCulloch 6 , Alberto Salleo 2 , Jarvist M Frost 1 , Alexander Giovannitti 2 , Jenny Nelson 1
Affiliation
|
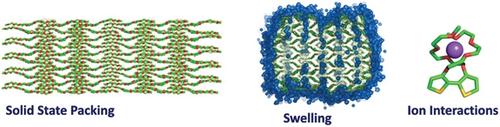
Exchanging hydrophobic alkyl-based side chains to hydrophilic glycol-based side chains is a widely adopted method for improving mixed-transport device performance, despite the impact on solid-state packing and polymer-electrolyte interactions being poorly understood. Presented here is a molecular dynamics (MD) force field for modeling alkoxylated and glycolated polythiophenes. The force field is validated against known packing motifs for their monomer crystals. MD simulations, coupled with X-ray diffraction (XRD), show that alkoxylated polythiophenes will pack with a “tilted stack” and straight interdigitating side chains, whilst their glycolated counterpart will pack with a “deflected stack” and an s-bend side-chain configuration. MD simulations reveal water penetration pathways into the alkoxylated and glycolated crystals—through the π-stack and through the lamellar stack respectively. Finally, the two distinct ways triethylene glycol polymers can bind to cations are revealed, showing the formation of a metastable single bound state, or an energetically deep double bound state, both with a strong side-chain length dependence. The minimum energy pathways for the formation of the chelates are identified, showing the physical process through which cations can bind to one or two side chains of a glycolated polythiophene, with consequences for ion transport in bithiophene semiconductors.
中文翻译:

侧链亲水性对氧-二噻吩半导体中的填充、溶胀和离子相互作用的影响
尽管对固态填充和聚合物-电解质相互作用的影响知之甚少,但将疏水性烷基侧链交换为亲水性二醇侧链是一种广泛采用的改善混合传输装置性能的方法。这里介绍的是用于模拟烷氧基化和乙二醇化聚噻吩的分子动力学 (MD) 力场。针对单体晶体的已知包装图案验证了力场。MD 模拟与 X 射线衍射 (XRD) 相结合,表明烷氧基化聚噻吩将包含“倾斜堆叠”和直交叉指状侧链,而它们的乙二醇化对应物将包含“偏转堆叠”和 s 弯曲侧链-链配置。MD 模拟揭示了水渗透进入烷氧基化和乙醇化晶体的途径——分别通过 π 叠层和层状叠层。最后,揭示了三甘醇聚合物与阳离子结合的两种不同方式,显示了亚稳态单键态或能量深度双键态的形成,两者都具有很强的侧链长度依赖性。确定了形成螯合物的最小能量路径,显示了阳离子可以与乙二醇化聚噻吩的一个或两个侧链结合的物理过程,从而对二噻吩半导体中的离子传输产生影响。或能量深的双束缚态,两者都具有很强的侧链长度依赖性。确定了形成螯合物的最小能量路径,显示了阳离子可以与乙二醇化聚噻吩的一个或两个侧链结合的物理过程,从而对二噻吩半导体中的离子传输产生影响。或能量深的双束缚态,两者都具有很强的侧链长度依赖性。确定了形成螯合物的最小能量路径,显示了阳离子可以与乙二醇化聚噻吩的一个或两个侧链结合的物理过程,从而对二噻吩半导体中的离子传输产生影响。
更新日期:2022-08-09
中文翻译:

侧链亲水性对氧-二噻吩半导体中的填充、溶胀和离子相互作用的影响
尽管对固态填充和聚合物-电解质相互作用的影响知之甚少,但将疏水性烷基侧链交换为亲水性二醇侧链是一种广泛采用的改善混合传输装置性能的方法。这里介绍的是用于模拟烷氧基化和乙二醇化聚噻吩的分子动力学 (MD) 力场。针对单体晶体的已知包装图案验证了力场。MD 模拟与 X 射线衍射 (XRD) 相结合,表明烷氧基化聚噻吩将包含“倾斜堆叠”和直交叉指状侧链,而它们的乙二醇化对应物将包含“偏转堆叠”和 s 弯曲侧链-链配置。MD 模拟揭示了水渗透进入烷氧基化和乙醇化晶体的途径——分别通过 π 叠层和层状叠层。最后,揭示了三甘醇聚合物与阳离子结合的两种不同方式,显示了亚稳态单键态或能量深度双键态的形成,两者都具有很强的侧链长度依赖性。确定了形成螯合物的最小能量路径,显示了阳离子可以与乙二醇化聚噻吩的一个或两个侧链结合的物理过程,从而对二噻吩半导体中的离子传输产生影响。或能量深的双束缚态,两者都具有很强的侧链长度依赖性。确定了形成螯合物的最小能量路径,显示了阳离子可以与乙二醇化聚噻吩的一个或两个侧链结合的物理过程,从而对二噻吩半导体中的离子传输产生影响。或能量深的双束缚态,两者都具有很强的侧链长度依赖性。确定了形成螯合物的最小能量路径,显示了阳离子可以与乙二醇化聚噻吩的一个或两个侧链结合的物理过程,从而对二噻吩半导体中的离子传输产生影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号