当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurements and Modeling of the Hydrate Phase Equilibrium Condition of Sour Gas Mixtures in the Presence of Salts and Alcohols
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-10 , DOI: 10.1021/acs.jced.2c00414 Liang Mu 1 , Qiqi Tan 1 , Xianlong Li 1 , Qingyun Zhang 1 , Qingyan Cui 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-08-10 , DOI: 10.1021/acs.jced.2c00414 Liang Mu 1 , Qiqi Tan 1 , Xianlong Li 1 , Qingyun Zhang 1 , Qingyan Cui 1
Affiliation
|
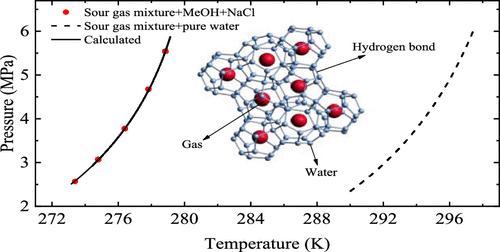
Sour natural gas can easily form clathrate hydrate and cause the pipeline blockage in transmission; salts and alcohols are usually added for shifting its phase boundary to inhibited regions. In this study, the hydrate equilibrium conditions of two (CH4 + CO2 + H2S + N2) quaternary gas mixtures (M1 and M2) in the NaCl solutions with and without methanol (MeOH) are measured using the isochoric pressure searching method. The results indicated that the hydrate phase boundary of sour gas mixtures mainly depended on its composition, where the content of H2S played a more important role than that of CO2. The presence of NaCl evidently decreased the hydrate equilibrium temperature of gas mixtures, while the temperature suppression effect did not present a linear relation to its concentration. Then, the 20 and 30 wt % MeOH was added to 5 wt % NaCl solution, for the CO2-rich (M1) and H2S-rich (M2) gas mixtures; the hydrate suppression temperatures reached 12.6 and 17.6 K, respectively. The measured data sets passed the thermodynamic consistency and have good reliability. A thermodynamic framework based on Chen-Guo model and UNIQUAC equation was proposed to predict the hydrate equilibrium condition of sour gas and mixtures in the aqueous solutions containing salts and alcohols. For the data sets measured in this work, our proposed model presented a smaller AADP (%) (average absolute deviation percent in pressure) than the commercial programs (PVTsim, Multiflash, CSMGem). For the literature data sets (116 points) on the sour gas and mixtures in the presence of alcohols [MeOH and ethylene glycol (EG)] and/or salts (NaCl, KCl, and CaCl2), this study also exhibited a higher accuracy than previous works.
中文翻译:

盐和醇存在下酸性气体混合物的水合物相平衡条件的测量和建模
含硫天然气易形成笼形水合物,造成输送管道堵塞;通常添加盐和醇以将其相界转移到抑制区域。在这项研究中,使用等容色谱法测量了含有和不含有甲醇 (MeOH) 的 NaCl 溶液中的两种 (CH 4 + CO 2 + H 2 S + N 2 ) 四元气体混合物 (M 1和 M 2 ) 的水合物平衡条件。压力搜索法。结果表明,酸性气体混合物的水合物相界主要取决于其组成,其中H 2 S的含量比CO 2的含量更重要。. NaCl的存在明显降低了气体混合物的水合物平衡温度,而温度抑制效应与其浓度不呈线性关系。然后,将 20 和 30 wt% 的 MeOH 添加到 5 wt% 的 NaCl 溶液中,以获得富含 CO 2 (M 1 ) 和富含H 2 S (M 2) 气体混合物;水合物抑制温度分别达到 12.6 和 17.6 K。实测数据组通过热力学一致性,具有良好的可靠性。提出了基于Chen-Guo模型和UNIQUAC方程的热力学框架来预测含盐和醇的水溶液中酸性气体及其混合物的水合物平衡条件。对于在这项工作中测量的数据集,我们提出的模型呈现出比商业程序(PVTsim、Multiflash、CSMGem)更小的 AADP (%)(压力的平均绝对偏差百分比)。对于在存在醇 [MeOH 和乙二醇 (EG)] 和/或盐 (NaCl、KCl 和 CaCl 2 ) 的情况下的酸性气体和混合物的文献数据集 (116 点),本研究还表现出更高的准确度比以前的作品。
更新日期:2022-08-10
中文翻译:

盐和醇存在下酸性气体混合物的水合物相平衡条件的测量和建模
含硫天然气易形成笼形水合物,造成输送管道堵塞;通常添加盐和醇以将其相界转移到抑制区域。在这项研究中,使用等容色谱法测量了含有和不含有甲醇 (MeOH) 的 NaCl 溶液中的两种 (CH 4 + CO 2 + H 2 S + N 2 ) 四元气体混合物 (M 1和 M 2 ) 的水合物平衡条件。压力搜索法。结果表明,酸性气体混合物的水合物相界主要取决于其组成,其中H 2 S的含量比CO 2的含量更重要。. NaCl的存在明显降低了气体混合物的水合物平衡温度,而温度抑制效应与其浓度不呈线性关系。然后,将 20 和 30 wt% 的 MeOH 添加到 5 wt% 的 NaCl 溶液中,以获得富含 CO 2 (M 1 ) 和富含H 2 S (M 2) 气体混合物;水合物抑制温度分别达到 12.6 和 17.6 K。实测数据组通过热力学一致性,具有良好的可靠性。提出了基于Chen-Guo模型和UNIQUAC方程的热力学框架来预测含盐和醇的水溶液中酸性气体及其混合物的水合物平衡条件。对于在这项工作中测量的数据集,我们提出的模型呈现出比商业程序(PVTsim、Multiflash、CSMGem)更小的 AADP (%)(压力的平均绝对偏差百分比)。对于在存在醇 [MeOH 和乙二醇 (EG)] 和/或盐 (NaCl、KCl 和 CaCl 2 ) 的情况下的酸性气体和混合物的文献数据集 (116 点),本研究还表现出更高的准确度比以前的作品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号