Gastroenterology ( IF 25.7 ) Pub Date : 2022-08-07 , DOI: 10.1053/j.gastro.2022.07.076 Jashodeep Datta 1 , Xizi Dai 2 , Anna Bianchi 2 , Iago De Castro Silva 2 , Siddharth Mehra 2 , Vanessa T Garrido 2 , Purushottam Lamichhane 3 , Samara P Singh 2 , Zhiqun Zhou 2 , Austin R Dosch 2 , Fanuel Messaggio 2 , Yuguang Ban 4 , Oliver Umland 5 , Peter J Hosein 6 , Nagaraj S Nagathihalli 1 , Nipun B Merchant 1
|
Background & Aims
We have shown that reciprocally activated rat sarcoma (RAS)/mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK) and Janus kinase/signal transducer and activator of transcription 3 (STAT3) pathways mediate therapeutic resistance in pancreatic ductal adenocarcinoma (PDAC), while combined MEK and STAT3 inhibition (MEKi+STAT3i) overcomes such resistance and alters stromal architecture. We now determine whether MEKi+STAT3i reprograms the cancer-associated fibroblast (CAF) and immune microenvironment to overcome resistance to immune checkpoint inhibition in PDAC.
Methods
CAF and immune cell transcriptomes in MEKi (trametinib)+STAT3i (ruxolitinib)-treated vs vehicle-treated Ptf1aCre/+;LSL-KrasG12D/+;Tgfbr2flox/flox (PKT) tumors were examined via single-cell RNA sequencing (scRNAseq). Clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats associated protein 9 silencing of CAF-restricted Map2k1/Mek1 or Stat3, or both, enabled interrogation of CAF-dependent effects on immunologic remodeling in orthotopic models. Tumor growth, survival, and immune profiling via mass cytometry by time-of-flight were examined in PKT mice treated with vehicle, anti-programmed cell death protein 1 (PD-1) monotherapy, and MEKi+STAT3i combined with anti-PD1.
Results
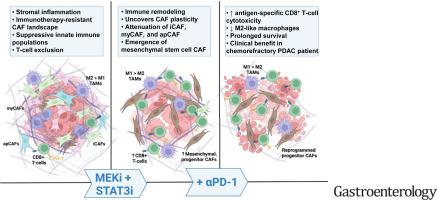
MEKi+STAT3i attenuates Il6/Cxcl1-expressing proinflammatory and Lrrc15-expressing myofibroblastic CAF phenotypes while enriching for Ly6a/Cd34-expressing CAFs exhibiting mesenchymal stem cell-like features via scRNAseq in PKT mice. This CAF plasticity is associated with M2-to-M1 reprogramming of tumor-associated macrophages, and enhanced trafficking of cluster of differentiation 8+ T cells, which exhibit distinct effector transcriptional programs. These MEKi+STAT3i-induced effects appear CAF-dependent, because CAF-restricted Mek1/Stat3 silencing mitigates inflammatory-CAF polarization and myeloid infiltration in vivo. Addition of MEKi+STAT3i to PD-1 blockade not only dramatically improves antitumor responses and survival in PKT mice but also augments recruitment of activated/memory T cells while improving their degranulating and cytotoxic capacity compared with anti–PD-1 monotherapy. Importantly, treatment of a patient who has chemotherapy-refractory metastatic PDAC with MEKi (trametinib), STAT3i (ruxolitinib), and PD-1 inhibitor (nivolumab) yielded clinical benefit.
Conclusions
Combined MEKi+STAT3i mitigates stromal inflammation and enriches for CAF phenotypes with mesenchymal stem cell-like properties to overcome immunotherapy resistance in PDAC.
中文翻译:

MEK 和 STAT3 联合抑制通过富集具有间充质干细胞样特征的癌症相关成纤维细胞来克服胰腺癌的免疫治疗耐药性,从而揭示基质可塑性
背景与目标
我们已经证明,相互激活的大鼠肉瘤 (RAS)/丝裂原激活蛋白激酶/细胞外信号调节激酶 (MEK) 和 Janus 激酶/信号转导和转录激活因子 3 (STAT3) 通路介导胰腺导管腺癌(PDAC)的治疗耐药),而 MEK 和 STAT3 联合抑制(MEKi+STAT3i)克服了这种阻力并改变了基质结构。我们现在确定 MEKi+STAT3i 是否会重新编程癌症相关成纤维细胞 (CAF) 和免疫微环境,以克服 PDAC 中对免疫检查点抑制的抵抗。
方法
MEKi (trametinib)+STAT3i (ruxolitinib) 治疗与媒介物治疗Ptf1a Cre/+治疗中的 CAF 和免疫细胞转录组; LSL-克拉斯G12D/+ ;通过单细胞 RNA 测序 (scRNAseq) 检查Tgfbr2 flox/flox (PKT) 肿瘤。成簇规则间隔的短回文重复序列/成簇规则间隔的短回文重复序列相关蛋白 9 CAF 限制性Map2k1 / Mek1或Stat3或两者的沉默,使得能够在原位模型中探究 CAF 依赖性对免疫重塑的影响。在接受媒介物、抗程序性细胞死亡蛋白 1 (PD-1) 单一疗法以及 MEKi+STAT3i 联合抗 PD1 治疗的 PKT 小鼠中,通过飞行时间质谱流式分析检查肿瘤生长、存活和免疫分析。
结果
MEKi+STAT3i 减弱表达IL6/Cxcl1的促炎细胞和表达Lrrc15的肌成纤维细胞 CAF 表型,同时通过 scRNAseq 富集表达Ly6a/Cd34的 CAF,从而在 PKT 小鼠中表现出间充质干细胞样特征。这种 CAF 可塑性与肿瘤相关巨噬细胞的 M2 到 M1 重编程以及分化 8 + T 细胞簇的运输增强有关,这些细胞表现出独特的效应转录程序。这些 MEKi+STAT3i 诱导的效应似乎依赖于 CAF,因为 CAF 限制的Mek1 / Stat3沉默减轻了体内炎症 CAF 极化和骨髓浸润。与抗 PD-1 单一疗法相比,在 PD-1 阻断中添加 MEKi+STAT3i 不仅可以显着改善 PKT 小鼠的抗肿瘤反应和存活率,还可以增加活化/记忆 T 细胞的募集,同时提高其脱粒和细胞毒性能力。重要的是,使用 MEKi(trametinib)、STAT3i(ruxolitinib)和 PD-1 抑制剂(nivolumab)治疗化疗难治性转移性 PDAC 患者产生了临床获益。
结论
MEKi+STAT3i 组合可减轻间质炎症并丰富具有间充质干细胞样特性的 CAF 表型,以克服 PDAC 中的免疫治疗耐药性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号