当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vacatadithiasapphyrins: Synthesis, Structure, and Spectral and Redox Properties
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-09 , DOI: 10.1021/acs.joc.2c01088 Md Ashif Ali 1 , Mangalampalli Ravikanth 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-09 , DOI: 10.1021/acs.joc.2c01088 Md Ashif Ali 1 , Mangalampalli Ravikanth 1
Affiliation
|
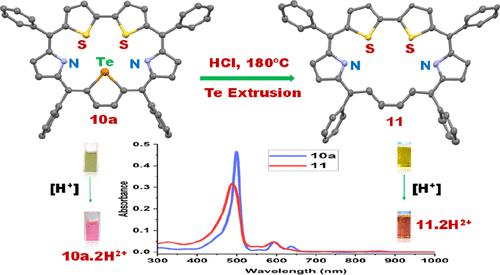
Four examples of novel aromatic [22]vacatadithiasapphyrins were synthesized by refluxing appropriate [22]telluradithiasapphyrins in 1,2-dichlorobenzene in the presence of excess HCl followed by simple column chromatographic purification. The [22]vacatadithiasapphyrins can exist in three conformers “in”, “out”, and “zigzag” w.r.t the butadiene moiety, and under our experimental conditions, the “out” conformer was the major compound. The X-ray structure obtained for one of the “out” conformers of vacatadithiasapphyrins revealed that the macrocycle was planar similar to its parent telluradithiasapphyrin and showed effective π-delocalization over the entire macrocyclic core. NMR studies supported the formation of the “out” conformer and suggested that the vacatadithiasapphyrins were less aromatic than the parent telluradithiasapphyrins. Density functional theory, time-dependent DFT, and nuclear independent chemical shift studies indicated that the protonated form of vacatadithiasapphyrin was more aromatic than the parent protonated telluradithiasapphyrin. The absorption spectra of vacatadithiasapphyrins showed a typical strong Soret band at ∼488 nm and four relatively broad Q-bands in the region of 590–860 nm, and electrochemical studies suggest that vacatadithiasapphyrins were easier to oxidize and easier to reduce compared to the parent telluradithiasapphyrins.
中文翻译:

Vacatadithiasapphyrins:合成、结构、光谱和氧化还原特性
通过在 1,2-二氯苯中在过量 HCl 的存在下回流适当的 [22] 碲二硫磷,然后进行简单的柱色谱纯化,合成了四个新型芳香族 [22] vacatadithiasappyrins 的例子。[22] vacatadithiasapphyrins 可以存在于丁二烯部分的“in”、“out”和“zigzag”三种构象异构体中,在我们的实验条件下,“out”构象异构体是主要化合物。为 vacatadithiasapphyrins 的“外”构象之一获得的 X 射线结构表明,大环是平面的,类似于其母体碲二硫菌素,并且在整个大环核心上显示出有效的 π-离域。NMR 研究支持“外”构象异构体的形成,并表明 vacatadithiasapphyrins 的芳香性低于母体碲二硫杂苷。密度泛函理论、时间依赖的 DFT 和与核无关的化学位移研究表明,质子化形式的 vacatadithiasapphyrin 比母体质子化的碲化物更芳香。vacatadithiasapphyrins的吸收光谱在~488 nm处显示出典型的强Soret带,在590-860 nm范围内显示出四个相对较宽的Q-带,电化学研究表明vacatadithiasapphyrins与母体碲二硫菌素相比更容易氧化和更容易还原.
更新日期:2022-08-09
中文翻译:

Vacatadithiasapphyrins:合成、结构、光谱和氧化还原特性
通过在 1,2-二氯苯中在过量 HCl 的存在下回流适当的 [22] 碲二硫磷,然后进行简单的柱色谱纯化,合成了四个新型芳香族 [22] vacatadithiasappyrins 的例子。[22] vacatadithiasapphyrins 可以存在于丁二烯部分的“in”、“out”和“zigzag”三种构象异构体中,在我们的实验条件下,“out”构象异构体是主要化合物。为 vacatadithiasapphyrins 的“外”构象之一获得的 X 射线结构表明,大环是平面的,类似于其母体碲二硫菌素,并且在整个大环核心上显示出有效的 π-离域。NMR 研究支持“外”构象异构体的形成,并表明 vacatadithiasapphyrins 的芳香性低于母体碲二硫杂苷。密度泛函理论、时间依赖的 DFT 和与核无关的化学位移研究表明,质子化形式的 vacatadithiasapphyrin 比母体质子化的碲化物更芳香。vacatadithiasapphyrins的吸收光谱在~488 nm处显示出典型的强Soret带,在590-860 nm范围内显示出四个相对较宽的Q-带,电化学研究表明vacatadithiasapphyrins与母体碲二硫菌素相比更容易氧化和更容易还原.







































 京公网安备 11010802027423号
京公网安备 11010802027423号